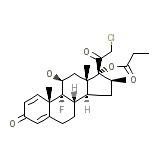
Olux




Olux Brand names, Olux Analogs
Olux Brand Names Mixture
- No information avaliable
Olux Chemical_Formula
C25H32ClFO5
Olux RX_link
http://www.rxlist.com/cgi/generic/olux.htm
Olux fda sheet
Olux msds (material safety sheet)
Olux Synthesis Reference
No information avaliable
Olux Molecular Weight
466.97 g/mol
Olux Melting Point
195.5-197 oC
Olux H2O Solubility
3.86 mg/L
Olux State
Solid
Olux LogP
3.588
Olux Dosage Forms
Foam (0.05%) and ointment (usp 0.05%, supplied in 15 g, 30 g, and 45 g tubes)
Olux Indication
For short-term topical treatment of the inflammatory and pruritic manifestations of moderate to severe corticosteroid-responsive dermatoses of the scalp.
Olux Pharmacology
Like other topical corticosteroids, clobetasol has anti-inflammatory, antipruritic, and vasoconstrictive properties. It is a very high potency topical corticosteroid that should not be used with occlusive dressings. It is recommended that treatment should be limited to 2 consecutive weeks and therapy should be discontinued when adequate results have been achieved.
Olux Absorption
Topical corticosteroids can be absorbed from intact healthy skin. The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusion, inflammation and/or other disease processes in the skin may also increase percutaneous absorption.
Olux side effects and Toxicity
Oral LD50 in rat and mouse is >3000 mg/kg. Topically applied clobetasol can be absorbed in sufficient amounts to produce systemic effects. Symptoms of overdose include thinning of skin and suppression of adrenal cortex (decreased ability to respond to stress).
Olux Patient Information
Olux Organisms Affected
Humans and other mammals