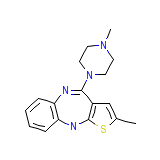
Olanzapine




Olanzapine Brand names, Olanzapine Analogs
Olanzapine Brand Names Mixture
- No information avaliable
Olanzapine Chemical_Formula
C17H20N4S
Olanzapine RX_link
http://www.rxlist.com/cgi/generic3/symbyax.htm
Olanzapine fda sheet
Olanzapine msds (material safety sheet)
Olanzapine Synthesis Reference
J. K. Chakrabarti et al., U.S. Pat. 5,229,382 (1991)
Olanzapine Molecular Weight
312.434 g/mol
Olanzapine Melting Point
195oC
Olanzapine H2O Solubility
No information avaliable
Olanzapine State
Solid
Olanzapine LogP
2.199
Olanzapine Dosage Forms
Tablet (oral)
Olanzapine Indication
For the treatment of schizophrenia and manic depression (bipolar disorder).
Olanzapine Pharmacology
Olanzapine, an atypical antipsychotic agent, is used to treat both negative and positive symptoms of schizophrenia, acute mania with bipolar disorder, agitation, and psychotic symptoms in dementia. Future uses may include the treatment of obsessive-compulsive disorder and severe behavioral disorders in autism. Structurally and pharmacologically similar to clozapine, olanzapine binds to alpha(1), dopamine, histamine H1, muscarinic, and serotonin type 2 (5-HT2) receptors.
Olanzapine Absorption
Well absorbed, with approximately 40% of the dose metabolized before reaching the systemic circulation.
Olanzapine side effects and Toxicity
No information avaliable
Olanzapine Patient Information
PATIENT INFORMATION
Physicians are advised to discuss the following issues with patients for whom they prescribe
olanzapine:
Orthostatic Hypotension: Patients should be advised of the risk of orthostatic hypotension,
especially during the period of initial dose titration and in association with the use of
concomitant drugs that may potentiate the orthostatic effect of olanzapine, e.g., diazepam
or alcohol.
Interference with Cognitive and Motor Performance: Because olanzapine has the potential to
impair judgment, thinking, or motor skills, patients should be cautioned about operating
hazardous machinery, including automobiles, until they are reasonably certain that olanzapine
therapy does not affect them adversely.
Pregnancy: Patients should be advised to notify their physician if they become pregnant or
intend to become pregnant during therapy with olanzapine.
Nursing: Patients should be advised not to breast-feed an infant if they are taking olanzapine.
Concomitant Medication: Patients should be advised to inform their physicians if they are taking,
or plan to take, any prescription or over-the-counter drugs, since there is a potential for
interactions.
Alcohol: Patients should be advised to avoid alcohol while taking olanzapine.
Heat Exposure and Dehydration: Patients should be advised regarding appropriate care in avoiding
overheating and dehydration.
Phenylketonurics: ZYPREXA ZYDIS (olanzapine orally disintegrating tablets) contains phenylalanine
(0.34, 0.45, 0.67, or 0.90 mg per 5, 10, 15, or 20mg tablet, respectively).
Laboratory Tests
Periodic assessment of transaminases is recommended in patients with significant hepatic disease.
Physicians are advised to discuss the following issues with patients for whom they prescribe
olanzapine:
Orthostatic Hypotension: Patients should be advised of the risk of orthostatic hypotension,
especially during the period of initial dose titration and in association with the use of
concomitant drugs that may potentiate the orthostatic effect of olanzapine, e.g., diazepam
or alcohol.
Interference with Cognitive and Motor Performance: Because olanzapine has the potential to
impair judgment, thinking, or motor skills, patients should be cautioned about operating
hazardous machinery, including automobiles, until they are reasonably certain that olanzapine
therapy does not affect them adversely.
Pregnancy: Patients should be advised to notify their physician if they become pregnant or
intend to become pregnant during therapy with olanzapine.
Nursing: Patients should be advised not to breast-feed an infant if they are taking olanzapine.
Concomitant Medication: Patients should be advised to inform their physicians if they are taking,
or plan to take, any prescription or over-the-counter drugs, since there is a potential for
interactions.
Alcohol: Patients should be advised to avoid alcohol while taking olanzapine.
Heat Exposure and Dehydration: Patients should be advised regarding appropriate care in avoiding
overheating and dehydration.
Phenylketonurics: ZYPREXA ZYDIS (olanzapine orally disintegrating tablets) contains phenylalanine
(0.34, 0.45, 0.67, or 0.90 mg per 5, 10, 15, or 20mg tablet, respectively).
Laboratory Tests
Periodic assessment of transaminases is recommended in patients with significant hepatic disease.
Olanzapine Organisms Affected
Humans and other mammals