Nufafloqo




Nufafloqo Brand names, Nufafloqo Analogs
- Akilen
- Baccidal
- Bactocin
- Danoflox
- Effexin
- Exocin
- Exocine
- Flobacin
- Flodemex
- Flotavid
- Flovid
- Floxal
- Floxil
- Floxin
- Floxin Otic
- Floxstat
- Fugacin
- Inoflox
- Kinflocin
- Kinoxacin
- Liflox
- Loxinter
- Marfloxacin
- Medofloxine
- Mergexin
- Novecin
- Nufafloqo
- O-Flox
- Obide
- Occidal
- Ocuflox
- Ofcin
- Oflin
- Oflocee
- Oflocet
- Oflocin
- Oflodal
- Oflodex
- Oflodura
- Oflox
- Ofloxin
- Ofus
- Onexacin
- Operan
- Orocin
- Otonil
- Pharflox
- Praxin
- Puiritol
- Qinolon
- Qipro
- Quinolon
- Quotavil
- Rilox
- Sinflo
- Tabrin
- Taravid
- Tariflox
- Tarivid
- Tarivid Eye Ear
- Tarivid Otic
- Telbit
- Tructum
- Uro Tarivid
- Viotisone
- Zanocin
Nufafloqo Brand Names Mixture
- No information avaliable
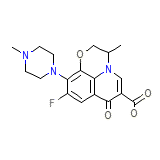
Nufafloqo Chemical_Formula
C18H20FN3O4
Nufafloqo RX_link
http://www.rxlist.com/cgi/generic/oflox.htm
Nufafloqo fda sheet
Nufafloqo msds (material safety sheet)
Nufafloqo Synthesis Reference
I. Hayakava et al., U.S. Pat. 4,382,892 (1983)
Nufafloqo Molecular Weight
361.368 g/mol
Nufafloqo Melting Point
250-257 oC
Nufafloqo H2O Solubility
28.3 mg/mL
Nufafloqo State
Solid
Nufafloqo LogP
1.268
Nufafloqo Dosage Forms
Solution (IV injection); Tablet; Opthalmic drops
Nufafloqo Indication
For the treatment of infections (respiratory tract, kidney, skin, soft tissue, UTI), urethral and cervical gonorrhoea.
Nufafloqo Pharmacology
Ofloxacin is a quinolone/fluoroquinolone antibiotic. Ofloxacin is bactericidal and its mode of action depends on blocking of bacterial DNA replication by binding itself to an enzyme called DNA gyrase, which allows the untwisting required to replicate one DNA double helix into two. Notably the drug has 100 times higher affinity for bacterial DNA gyrase than for mammalian. Ofloxacin is a broad-spectrum antibiotic that is active against both Gram-positive and Gram-negative bacteria.
Nufafloqo Absorption
Bioavailability of ofloxacin in the tablet formulation is approximately 98%
Nufafloqo side effects and Toxicity
LD50=5450 mg/kg (orally in mice)
Nufafloqo Patient Information
PATIENT INFORMATION
Patients should be advised:
- to drink fluids liberally;
- that mineral supplements, vitamins with iron or minerals, calcium- , aluminum-, or magnesium-based
antacids, sucralfate or Videx�, (Didanosine), chewable/buffered tablets or the pediatric powder for
oral solution should not be taken within the two-hour period before or within the two-hour period after
taking ofloxacin (See Drug Interactions);
- that ofloxacin can be taken without regard to meals;
- that ofloxacin may cause neurologic adverse effects (e. g. , dizziness, lightheadedness) and that
patients should know how they react to ofloxacin before they operate an automobile or machinery or
engage in activities requiring mental alertness and coordination
- to discontinue treatment and inform their physician if they experience pain, inflammation, or rupture
of a tendon, and to rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture
has been confidently excluded;
- that ofloxacin may be associated with hypersensitivity reactions, even following the first dose, to
discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat,
difficulty in swallowing or breathing, any swelling suggesting angioedema (e. g. , swelling of the lips,
tongue, face; tightness of the throat, hoarseness), or any other symptom of an allergic reaction
- to avoid excessive sunlight or artificial ultraviolet light while receiving ofloxacin and to discontinue
therapy if phototoxicity (e. g. , skin eruption) occurs;
- that if they are diabetic and are being treated with insulin or an oral hypoglycemic drug, to discontinue
ofloxacin immediately if a hypoglycemic reaction occurs and consult a physician
- that convulsions have been reported in patients taking quinolones, including ofloxacin, and to notify their
physician before taking this drug if there is a history of this condition.
Patients should be advised:
- to drink fluids liberally;
- that mineral supplements, vitamins with iron or minerals, calcium- , aluminum-, or magnesium-based
antacids, sucralfate or Videx�, (Didanosine), chewable/buffered tablets or the pediatric powder for
oral solution should not be taken within the two-hour period before or within the two-hour period after
taking ofloxacin (See Drug Interactions);
- that ofloxacin can be taken without regard to meals;
- that ofloxacin may cause neurologic adverse effects (e. g. , dizziness, lightheadedness) and that
patients should know how they react to ofloxacin before they operate an automobile or machinery or
engage in activities requiring mental alertness and coordination
- to discontinue treatment and inform their physician if they experience pain, inflammation, or rupture
of a tendon, and to rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture
has been confidently excluded;
- that ofloxacin may be associated with hypersensitivity reactions, even following the first dose, to
discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat,
difficulty in swallowing or breathing, any swelling suggesting angioedema (e. g. , swelling of the lips,
tongue, face; tightness of the throat, hoarseness), or any other symptom of an allergic reaction
- to avoid excessive sunlight or artificial ultraviolet light while receiving ofloxacin and to discontinue
therapy if phototoxicity (e. g. , skin eruption) occurs;
- that if they are diabetic and are being treated with insulin or an oral hypoglycemic drug, to discontinue
ofloxacin immediately if a hypoglycemic reaction occurs and consult a physician
- that convulsions have been reported in patients taking quinolones, including ofloxacin, and to notify their
physician before taking this drug if there is a history of this condition.
Nufafloqo Organisms Affected
Enteric bacteria and other eubacteria