Nizoral a-D




Nizoral a-D Brand names, Nizoral a-D Analogs
Nizoral a-D Brand Names Mixture
- No information avaliable
Nizoral a-D Chemical_Formula
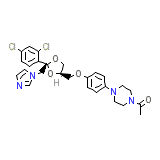
C26H28Cl2N4O4
Nizoral a-D RX_link
http://www.rxlist.com/cgi/generic/ketocon.htm
Nizoral a-D fda sheet
Nizoral a-D msds (material safety sheet)
Nizoral a-D Synthesis Reference
J. Heeres et al., U.S. Pat. 4,144,346 (1979)
Nizoral a-D Molecular Weight
531.43 g/mol
Nizoral a-D Melting Point
146 oC
Nizoral a-D H2O Solubility
0.0866 mg/L
Nizoral a-D State
Solid
Nizoral a-D LogP
3.651
Nizoral a-D Dosage Forms
Tablets; Shampoo; Cream; Suspension
Nizoral a-D Indication
For the treatment of the following systemic fungal infections: candidiasis, chronic mucocutaneous candidiasis, oral thrush, candiduria, blastomycosis, coccidioidomycosis, histoplasmosis, chromomycosis, and paracoccidioidomycosis.
Nizoral a-D Pharmacology
Ketoconazole, like clotrimazole, fluconazole, itraconazole, and miconazole, is an imidazole antifungal agent.
Nizoral a-D Absorption
Moderate
Nizoral a-D side effects and Toxicity
Hepatotoxicity, LD50=86 mg/kg (orally in rat)
Nizoral a-D Patient Information
Shampoo: May be irritating to mucous membranes of the eyes and contact with this area should be avoided.
There have been reports that use of the shampoo resulted in removal of the curl from permanently waved hair.
Tablet: Patients should be instructed to report any signs and symptoms which may suggest liver dysfunction so that appropriate biochemical testing can be done. Such signs and symptoms may include unusual fatigue, anorexia, nausea and/or vomiting, jaundice, dark urine or pale stools
Nizoral a-D Organisms Affected
Fungi