Nibrol




Nibrol Brand names, Nibrol Analogs
- Algosediv
- Asidon 3
- Asmadion
- Asmaval
- Bonbrain
- Bonbrrin
- Calmore
- Calmorex
- Contergan
- Corronarobetin
- Distaval
- Distaxal
- Distoval
- Ectiluran
- Enterosediv
- Gastrinide
- Glupan
- Glutanon
- Grippex
- Hippuzon
- Imida-Lab
- Imidan
- Imidene
- Isomin
- Kedavon
- Kevadon
- Lulamin
- N-Phthalimidoglutamic acid imide
- N-Phthaloylglutamimide
- N-Phthalylglutamic acid imide
- Neaufatin
- Neo
- Neosedyn
- Neosydyn
- Nerosedyn
- Neufatin
- Neurodyn
- Neurosedin
- Neurosedym
- Neurosedyn
- Nevrodyn
- Nibrol
- Noctosediv
- Noxodyn
- Pangul
- Pantosediv
- Poly-Giron
- Polygripan
- Predni-Sediv
- Pro-ban M
- Profarmil
- Psycholiquid
- Psychotablets
- Quetimid
- Quietoplex
- Sandormin
- Sedalis
- Sedalis sedi-lab
- Sedimide
- Sedin
- Sedisperil
- Sedoval
- Shin-naito S
- Shinnibrol
- Sleepan
- Slipro
- Softenil
- Softenon
- THALOMID
- Talargan
- Talimol
- Talismol
- Telagan
- Telargan
- Telargean
- Tensival
- Thalidomide 99%
- Thalidomine USP26
- Thalin
- Thalinette
- Theophilcholine
- Ulcerfen
- Valgis
- Valgraine
- Yodomin
- alpha-phthalimidoglutarimide
Nibrol Brand Names Mixture
- No information avaliable
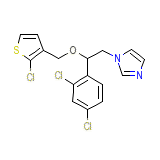
Nibrol Chemical_Formula
C16H13Cl3N2OS
Nibrol RX_link
http://www.rxlist.com/cgi/generic3/tioconaz.htm
Nibrol fda sheet
Nibrol msds (material safety sheet)
Nibrol Synthesis Reference
No information avaliable
Nibrol Molecular Weight
387.711 g/mol
Nibrol Melting Point
No information avaliable
Nibrol H2O Solubility
No information avaliable
Nibrol State
Solid
Nibrol LogP
4.971
Nibrol Dosage Forms
Cream; Ointment; Suppository (each applicator-full provides approximately 4.6 grams of ointment containing 300 mg of tioconazole)
Nibrol Indication
For the local treatment of vulvovaginal candidiasis (moniliasis).
Nibrol Pharmacology
Tioconazole is a broad-spectrum imidazole antifungal agent that inhibits the growth of human pathogenic yeasts. Tioconazole exhibits fungicidal activity in vitro against Candida albicans, other species of the genus Candida, and against Torulopsis glabrata. Tioconazole prevents the growth and function of some fungal organisms by interfering with the production of substances needed to preserve the cell membrane. This drug is effective only for infections caused by fungal organisms. It will not work for bacterial or viral infections.
Nibrol Absorption
Systemic absorption following a single intravaginal application of tioconazole in nonpregnant patients is negligible.
Nibrol side effects and Toxicity
Symptoms of overdose include erythema, stinging, blistering, peeling, edema, pruritus, urticaria, burning,
Nibrol Patient Information
Nibrol Organisms Affected
Yeast and other fungi