Nexium Iv




Nexium Iv Brand names, Nexium Iv Analogs
Nexium Iv Brand Names Mixture
- No information avaliable
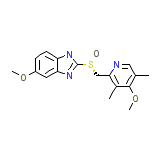
Nexium Iv Chemical_Formula
C17H19N3O3S
Nexium Iv RX_link
http://www.rxlist.com/cgi/generic3/esomeprazole.htm
Nexium Iv fda sheet
Nexium Iv msds (material safety sheet)
Nexium Iv Synthesis Reference
No information avaliable
Nexium Iv Molecular Weight
345.417 g/mol
Nexium Iv Melting Point
155 oC
Nexium Iv H2O Solubility
Very slightly soluble in water
Nexium Iv State
Solid
Nexium Iv LogP
1.078
Nexium Iv Dosage Forms
Tablet (delayed-release); Delayed-release capsules; IV injection solution; IV iufusion solution
Nexium Iv Indication
For the treatment of acid-reflux disorders (GERD) and peptic ulcer disease
Nexium Iv Pharmacology
Esomeprazole is a compound that inhibits gastric acid secretion and is indicated in the treatment of gastroesophageal reflux disease (GERD), the healing of erosive esophagitis, and H. pylori eradication to reduce the risk of duodenal ulcer recurrence. Esomeprazole belongs to a new class of antisecretory compounds, the substituted benzimidazoles, that do not exhibit anticholinergic or H2 histamine antagonistic properties, but that suppress gastric acid secretion by specific inhibition of the H+/K+ ATPase enzyme system at the secretory surface of the gastric parietal cell. Because this enzyme system is regarded as the acid (proton) pump within the gastric mucosa, Esomeprazole has been characterized as a gastric acid-pump inhibitor, in that it blocks the final step of acid production. This effect is dose-related and leads to inhibition of both basal and stimulated acid secretion irrespective of the stimulus.
Nexium Iv Absorption
90%
Nexium Iv side effects and Toxicity
Blurred vision, confusion, drowsiness, dry mouthflushingheadache, nausea, rapid heartbeat, sweating
Nexium Iv Patient Information
No information avaliable
Nexium Iv Organisms Affected
Humans and other mammals