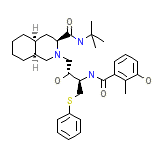
Nelfinavir




Nelfinavir Brand names, Nelfinavir Analogs
Nelfinavir Brand Names Mixture
- No information avaliable
Nelfinavir Chemical_Formula
C32H45N3O4S
Nelfinavir RX_link
http://www.rxlist.com/cgi/generic2/nelfin.htm
Nelfinavir fda sheet
Nelfinavir msds (material safety sheet)
Nelfinavir Synthesis Reference
No information avaliable
Nelfinavir Molecular Weight
567.784 g/mol
Nelfinavir Melting Point
349.84 oC
Nelfinavir H2O Solubility
Slightly soluble
Nelfinavir State
Solid
Nelfinavir LogP
5.247
Nelfinavir Dosage Forms
Tablet (oral - 250 mg, 625 mg); Powder (oral)
Nelfinavir Indication
Used in combination with other antiviral drugs in the treatment of HIV in both adults and children.
Nelfinavir Pharmacology
Nelfinavir is a protease inhibitor with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Protease inhibitors block the part of HIV called protease. HIV-1 protease is an enzyme required for the proteolytic cleavage of the viral polyprotein precursors into the individual functional proteins found in infectious HIV-1. Nelfinavir binds to the protease active site and inhibits the activity of the enzyme. This inhibition prevents cleavage of the viral polyproteins resulting in the formation of immature non-infectious viral particles. Protease inhibitors are almost always used in combination with at least two other anti-HIV drugs.
Nelfinavir Absorption
Well absorbed following oral administration.
Nelfinavir side effects and Toxicity
Oral LD50 is over 5g/kg in rats. Side effects include thirst and hunger, unexplained weight loss, increased urination, fatigue, and dry, itchy skin.
Nelfinavir Patient Information
PATIENT INFORMATION
Patients should be informed that VIRACEPT is not a cure for HIV infection and that they may
continue to acquire illnesses associated with advanced HIV infection, including opportunistic
infections.
Patients should be told that the long-term effects of VIRACEPT are unknown at this time. They
should be told that there is currently no data demonstrating that VIRACEPT therapy can reduce
the risk of transmitting HIV to others through sexual contact or blood contamination.
Patients should be advised to take VIRACEPT every day as prescribed. Patients should not alter
the dose or discontinue therapy without consulting with their doctor. If a dose is missed, patients
should take the dose as soon as possible and then return to their normal schedule. However, if a dose
is skipped, the patient should not double the next dose.
The most frequent adverse event associated with VIRACEPT is diarrhea, which can usually be controlled
with non-prescription drugs, such as loperamide, which slow gastrointestinal motility.
VIRACEPT may interact with some drugs, therefore, patients should be advised to report to their doctor
the use of any other prescription or non-prescription medication.
Patients receiving oral contraceptives should be instructed that alternate or additional contraceptive
measures should be used during therapy with VIRACEPT.
Patients should be informed that VIRACEPT is not a cure for HIV infection and that they may
continue to acquire illnesses associated with advanced HIV infection, including opportunistic
infections.
Patients should be told that the long-term effects of VIRACEPT are unknown at this time. They
should be told that there is currently no data demonstrating that VIRACEPT therapy can reduce
the risk of transmitting HIV to others through sexual contact or blood contamination.
Patients should be advised to take VIRACEPT every day as prescribed. Patients should not alter
the dose or discontinue therapy without consulting with their doctor. If a dose is missed, patients
should take the dose as soon as possible and then return to their normal schedule. However, if a dose
is skipped, the patient should not double the next dose.
The most frequent adverse event associated with VIRACEPT is diarrhea, which can usually be controlled
with non-prescription drugs, such as loperamide, which slow gastrointestinal motility.
VIRACEPT may interact with some drugs, therefore, patients should be advised to report to their doctor
the use of any other prescription or non-prescription medication.
Patients receiving oral contraceptives should be instructed that alternate or additional contraceptive
measures should be used during therapy with VIRACEPT.
Nelfinavir Organisms Affected
Human immunodeficiency virus