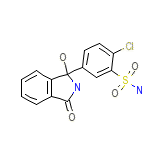
Natriuran




Natriuran Brand names, Natriuran Analogs
Natriuran Brand Names Mixture
- Tenoretic (chlorthalidone + atenolol)
Natriuran Chemical_Formula
C14H11ClN2O4S
Natriuran RX_link
http://www.rxlist.com/cgi/generic3/chlorthalidone.htm
Natriuran fda sheet
Natriuran msds (material safety sheet)
Natriuran Synthesis Reference
Graf et al.; Helv. Chem.; Acta 42; 1085 (1959); U.S.Pat.3,055,904 (1962)
Natriuran Molecular Weight
338.767 g/mol
Natriuran Melting Point
218-264oC
Natriuran H2O Solubility
120 mg/L
Natriuran State
Solid
Natriuran LogP
1.718
Natriuran Dosage Forms
Tablet
Natriuran Indication
For management of hypertension either as the sole therapeutic agent or to enhance the effect of other antihypertensive drugs in the more severe forms of hypertension.
Natriuran Pharmacology
Chlorthalidone, a monosulfonamyl diuretic, differs form other thiazide diuretics in that a double ring system is incorporated into its structure. Chlorthalidone is used alone or with atenolol in the management of hypertension and edema.
Natriuran Absorption
Absorbed relatively rapidly after oral administration.
Natriuran side effects and Toxicity
Symptoms of overdose include nausea, weakness, dizziness and disturbances of electrolyte balance.
Natriuran Patient Information
Patients should inform their physician if they have: (1) had an allergic reaction to chlorthalidone or other diuretics or have asthma, (2) kidney disease, (3) liver disease, (4) gout, (5) systemic lupus erythematosus, or (6) been taking other drugs such as cortisone, digitalis, lithium carbonate, or drugs for diabetes.
Patients should be cautioned to contact their physician if they experience any of the following symptoms of potassium loss: excess thirst, tiredness, drowsiness, restlessness, muscle pains or cramps, nausea, vomiting, or increased heart rate or pulse.
Patients should also be cautioned that taking alcohol can increase the chance of dizziness occurring.
Natriuran Organisms Affected
Humans and other mammals