Nabac 25 Ec




Nabac 25 Ec Brand names, Nabac 25 Ec Analogs
- AT-7
- Acigena
- Almederm
- Bilevon
- Bivelon
- Compound G-11
- Cotofilm
- Dermadex
- Dial
- Distodin
- E-Z Scrub
- Eleven
- Exofene
- Fascol
- Fesia-Sin
- Fomac
- Fostril
- G-Eleven
- G-II
- Gamophen
- Gamophene
- Germa-Medica
- HCP
- Hexa-Germ
- Hexabalm
- Hexachlorofen
- Hexachlorophane
- Hexachlorophen
- Hexachlorophene, Pharma
- Hexafen
- Hexascrub
- Hexazinone
- Hexide
- Hexophene
- Hexosan
- Isobac
- Isobac 20
- Nabac
- Nabac 25 Ec
- Neosept V
- Phiso-Scrub
- Phisodan
- Phisohex
- Pre-Op
- Rcra Waste Number U132
- Ritosept
- Scrubteam Surgical Spongebrush
- Septi-Soft
- Septisol
- Septofen
- Soy-Dome
- Ster-Zac
- Steral
- Steraskin
- Surgi-Cen
- Surgi-Cin
- Surofene
- Tersaseptic
- Trichlorophene
- Turgex
Nabac 25 Ec Brand Names Mixture
- Fosteen Dermatologic Shampoo (Hexachlorophene + Lanolin + Salicylic Acid + Sulfur)
- Hexaphenyl (Hexachlorophene + Isopropyl Alcohol)
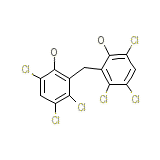
Nabac 25 Ec Chemical_Formula
C13H6Cl6O2
Nabac 25 Ec RX_link
http://www.rxlist.com/cgi/generic2/hexchlorph.htm
Nabac 25 Ec fda sheet
Nabac 25 Ec msds (material safety sheet)
Nabac 25 Ec Synthesis Reference
No information avaliable
Nabac 25 Ec Molecular Weight
406.902 g/mol
Nabac 25 Ec Melting Point
166.5 oC (boiling point)
Nabac 25 Ec H2O Solubility
140 mg/L
Nabac 25 Ec State
Liquid
Nabac 25 Ec LogP
6.941
Nabac 25 Ec Dosage Forms
Liquid
Nabac 25 Ec Indication
For use as a surgical scrub and a bacteriostatic skin cleanser. It may also be used to control an outbreak of gram-positive infection where other infection control procedures have been unsuccessful.
Nabac 25 Ec Pharmacology
Hexachlorophene, a detergent cleanser, is an antibacterial sudsing emulsion for topical administration. It is a bacteriostatic cleansing agent. It cleanses the skin thoroughly and has bacteriostatic action against staphylococci and other gram-positive bacteria. Cumulative antibacterial action develops with repeated use. Cleansing with alcohol or soaps containing alcohol removes the antibacterial residue.
Nabac 25 Ec Absorption
Detectable blood levels of hexachlorophene following absorption through intact skin have been found in subjects who regularly scrubbed with hexachlorophene.
Nabac 25 Ec side effects and Toxicity
Oral, rat LD50: 66 mg/kg. Signs of overdose include anorexia, vomiting, abdominal cramps, diarrhea, dehydration, convulsions, hypotension, and shock, and in several reported instances, fatalities.
Nabac 25 Ec Patient Information
No information avaliable
Nabac 25 Ec Organisms Affected
Enteric bacteria and other eubacteria