Mylproin




Mylproin Brand names, Mylproin Analogs
Mylproin Brand Names Mixture
- No information avaliable
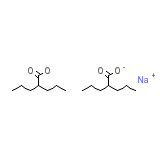
Mylproin Chemical_Formula
Mylproin RX_link
Mylproin fda sheet
Mylproin msds (material safety sheet)
Mylproin Synthesis Reference
Mylproin Molecular Weight
Mylproin Melting Point
Mylproin H2O Solubility
Mylproin State
Mylproin LogP
Mylproin Dosage Forms
Mylproin Indication
Mylproin Pharmacology
Mylproin Absorption
Mylproin side effects and Toxicity
Mylproin Patient Information
Important Information for Women Who Could Become Pregnant About the Use of DEPAKOTE® (divalproex sodium) Tablets
Please read this leaflet carefully before you take DEPAKOTE® (divalproex sodium) tablets. This leaflet provides a summary of important information about taking DEPAKOTE to women who could become pregnant. If you have any questions or concerns,or want more information about DEPAKOTE, contact your doctor or pharmacist.
Information For Women Who Could Become Pregnant
DEPAKOTE can be obtained only by prescription from your doctor. The decision to use DEPAKOTE is one that you and your doctor should make together, taking into account your individual needs and medical condition.
Before using DEPAKOTE, women who can become pregnant should consider the fact that DEPAKOTE has been associated with birth defects, in particular, with spina bifida and other defects related to failure of the spinal canal to close normally. Approximately 1 to 2% of children born to women with epilepsy taking DEPAKOTE in the first 12 weeks of pregnancy had these defects (based on data from the Centers for Disease Control, a U.S. agency based in Atlanta). The incidence in the general population is 0.1 to 0.2%.
Information For Women Who Are Planning to Get Pregnant
ï Women taking DEPAKOTE who are planning to get pregnant should discuss the treatment options with their doctor.
Information For Women Who Become Pregnant While Taking DEPAKOTE
ï If you become pregnant while taking DEPAKOTE you should contact your doctor immediately.
Other Important Information About DEPAKOTE Tablets
ï DEPAKOTE tablets should be taken exactly as it is prescribed by your doctor to get the most benefits from DEPAKOTE and reduce the risk of side effects.
ï If you have taken more than the prescribed dose of DEPAKOTE, contact your hospital emergency room or local poison center immediately.
ï This medication was prescribed for your particular condition. Do not use it for another condition or give the drug to others.
Facts About Birth Defects
It is important to know that birth defects may occur even in children of individuals not taking any medications or without any additional risk factors.
This summary provides important information about the use of DEPAKOTE to women who could become pregnant. If you would like more information about the other potential risks and benefits of DEPAKOTE, ask your doctor or pharmacist to let you read the professional labeling and then discuss it with them. If you have any questions or concerns about taking DEPAKOTE, you should discuss them with your doctor.