Mykrox




Mykrox Brand names, Mykrox Analogs
Mykrox Brand Names Mixture
- No information avaliable
Mykrox Chemical_Formula
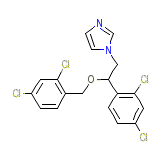
C18H14Cl4N2O
Mykrox RX_link
http://www.rxlist.com/cgi/generic2/micon.htm
Mykrox fda sheet
Mykrox msds (material safety sheet)
Mykrox Synthesis Reference
No information avaliable
Mykrox Molecular Weight
416.127 g/mol
Mykrox Melting Point
159 - 163 oC
Mykrox H2O Solubility
1g/100mL (20°C)
Mykrox State
Solid
Mykrox LogP
6.02
Mykrox Dosage Forms
Topical cream (2%); Vaginal cream (2%); Vaginal suppositories (100mg, 200mg)
Mykrox Indication
For topical application in the treatment of tinea pedis (athlete’s foot), tinea cruris, and tinea corporis caused by Trichophyton rubrum, Trichophyton mentagrophytes, and Epidermophyton floccosum, in the treatment of cutaneous candidiasis (moniliasis), and in the treatment of tinea versicolor.
Mykrox Pharmacology
Miconazole is an anti-fungal medication related to fluconazole (Diflucan), ketoconazole (Nizoral), itraconazole (Sporanox), and clotrimazole (Lotrimin, Mycelex). It is used either on the skin or in the vagina for fungal infections. Miconazole was approved by the FDA in 1974. Miconazole prevents fungal organisms from producing vital substances required for growth and function. This medication is effective only for infections caused by fungal organisms. It will not work for bacterial or viral infections.
Mykrox Absorption
No information avaliable
Mykrox side effects and Toxicity
Oral, mouse: LD50 = 3800 mg/kg; Oral, rat: LD50 = 3 gm/kg. Ingestion of the amounts of the components contained in a tube of cream are unlikely to produce overdosage and toxic effects.
Mykrox Patient Information
No information avaliable
Mykrox Organisms Affected
Fungi, yeast and protozoans