Monistat IV




Monistat IV Brand names, Monistat IV Analogs
- Benzoylmetronildazole
- Daktarin
- Femizol-M
- M-zole 3 Combination Pack
- M-zole 7 Dual Pack
- MCZ
- Micatin
- Miconazole 3
- Miconazole 3 Combination Pack
- Miconazole 7
- Miconazole 7 Combination Pack
- Miconazole Nitrate
- Miconazole Nitrate Combination Pack
- Miconazole-7
- Micozole
- Minostate
- Monazole 7
- Monistat
- Monistat 1 Combination Pack
- Monistat 3
- Monistat 3 Combination Pack
- Monistat 3 Dual-Pak
- Monistat 3 Vaginal Ovules
- Monistat 5
- Monistat 5 Tampon
- Monistat 7
- Monistat 7 Combination Pack
- Monistat 7 Dual-Pak
- Monistat 7 Vaginal Suppositories
- Monistat Dual- PAK
- Monistat IV
- Monistat-3 Combination Pack
- Monistat-Derm
- Novo-Miconazole Vaginal Ovules
- Zeasorb-AF
Monistat IV Brand Names Mixture
- No information avaliable
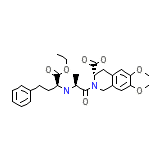
Monistat IV Chemical_Formula
C27H34N2O7
Monistat IV RX_link
http://www.rxlist.com/cgi/generic2/moexip.htm
Monistat IV fda sheet
Monistat IV msds (material safety sheet)
Monistat IV Synthesis Reference
No information avaliable
Monistat IV Molecular Weight
498.568 g/mol
Monistat IV Melting Point
No information avaliable
Monistat IV H2O Solubility
Soluble (about 10% weight-to-volume) in distilled water at room temperature as HCl salt.
Monistat IV State
Solid
Monistat IV LogP
3.31
Monistat IV Dosage Forms
Tablets (scored, coated, containing 7.5 mg or 15 mg of moexipril hydrochloride for oral administration)
Monistat IV Indication
For treatment of patients with hypertension.
Monistat IV Pharmacology
Moexipril is a non-sulfhydryl containing precursor of the active angiotensin-converting enzyme (ACE) inhibitor moexiprilat. It is used to treat high blood pressure (hypertension). It works by relaxing blood vessels, causing them to widen. Lowering high blood pressure helps prevent strokes, heart attacks and kidney problems.
Monistat IV Absorption
Moexipril is incompletely absorbed, with bioavailability as moexiprilat of about 13% compared to intravenous (I.V.) moexipril (both measuring the metabolite moexiprilat), and is markedly affected by food, which reduces Cmax and AUC by about 70% and 40% respectively after the ingestion of a low-fat breakfast or by 80% and 50% respectively after the ingestion of a high-fat breakfast.
Monistat IV side effects and Toxicity
Human overdoses of moexipril have not been reported. In case reports of overdoses with other ACE inhibitors, hypotension has been the principal adverse effect noted. Single oral doses of 2 g/kg moexipril were associated with significant lethality in mice. Rats, however, tolerated single oral doses of up to 3 g/kg.
Monistat IV Patient Information
Monistat IV Organisms Affected
Humans and other mammals