Moexipril HCL




Moexipril HCL Brand names, Moexipril HCL Analogs
Moexipril HCL Brand Names Mixture
- No information avaliable
Moexipril HCL Chemical_Formula
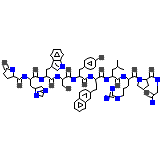
C66H83N17O13
Moexipril HCL RX_link
http://www.rxlist.com/cgi/generic2/nafarelin.htm
Moexipril HCL fda sheet
Moexipril HCL msds (material safety sheet)
Moexipril HCL Synthesis Reference
No information avaliable
Moexipril HCL Molecular Weight
1321.6344 g/mol
Moexipril HCL Melting Point
No information avaliable
Moexipril HCL H2O Solubility
No information avaliable
Moexipril HCL State
Solid
Moexipril HCL LogP
No information avaliable
Moexipril HCL Dosage Forms
Liquid; Metered-dose (aerosol)
Moexipril HCL Indication
For treatment of gonadotropin-dependent precocious puberty in children of both sexes and for the treatment of endometriosis.
Moexipril HCL Pharmacology
Nafarelin is a potent agonistic analog of gonadotropin-releasing hormone (GnRH). At the onset of administration, nafarelin stimulates the release of the pituitary gonadotropins, LH and FSH, resulting in a temporary increase of gonadal steroidogenesis. Repeated dosing abolishes the stimulatory effect on the pituitary gland. Twice daily administration leads to decreased secretion of gonadal steroids by about 4 weeks; consequently, tissues and functions that depend on gonadal steroids for their maintenance become quiescent. After nafarelin therapy is discontinued, pituitary and ovarian function normalize and estradiol serum concentrations increase to pretreatment levels. Recurrences of endometriosis are frequent after cessation of any hormonal therapy and after surgery that leaves the ovaries and/or uterus intact.
Moexipril HCL Absorption
Rapidly absorbed into the systemic circulation after intranasal administration. Bioavailability from a 400 µg dose averaged 2.8% (range 1.2 to 5.6%). Not absorbed after oral administration.
Moexipril HCL side effects and Toxicity
In experimental animals, a single subcutaneous administration of up to 60 times the recommended human dose (on a µg/kg basis, not adjusted for bioavailability) had no adverse effects. At present, there is no clinical evidence of adverse effects following overdosage of GnRH analogs.
Moexipril HCL Patient Information
Moexipril HCL Organisms Affected
Humans and other mammals