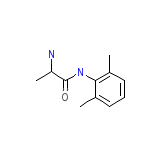
Mintezol




Mintezol Brand names, Mintezol Analogs
- Apl-Luster
- Arbotect
- Bioguard
- Bovizole
- Chemviron TK 100
- Cropasal
- Drawipas
- Eprofil
- Equivet TZ
- Equizole
- Hokustar hp
- Hymush
- Lombristop
- Mertec
- Mertect
- Mertect 160
- Mertect 340f
- Mertect lsp
- Metasol TK 100
- Metasol TK 10
- Mintesol
- Mintezol
- Minzolum
- Mycozol
- Nemacin
- Nemapan
- Omnizole
- Ormogal
- Polival
- RPH
- Sanaizol 100
- Sistesan
- Storite
- TBZ 6
- TBZ 60W
- TBDZ
- TBZ
- Tebuzate
- Tecto
- Tecto 10P
- Tecto 40F
- Tecto 60
- Tecto B
- Tecto rph
- Testo
- Thiaben
- Thiabendazol
- Thiabendole
- Thiabenzazole
- Thiabenzole
- Thibendole
- Thibenzol
- Thibenzole
- Thibenzole 200
- Thibenzole att
- Thiprazole
- Tiabenda
- Tiabendazol
- Tiabendazole
- Tibimix 20
- Tobaz
- Top form wormer
- Triasox
- Tubazole
Mintezol Brand Names Mixture
- No information avaliable
Mintezol Chemical_Formula
C11H16N2O
Mintezol RX_link
http://www.rxlist.com/cgi/generic3/tocainide.htm
Mintezol fda sheet
Mintezol msds (material safety sheet)
Mintezol Synthesis Reference
No information avaliable
Mintezol Molecular Weight
192.258 g/mol
Mintezol Melting Point
246-266 oC
Mintezol H2O Solubility
1.07E+004 mg/L
Mintezol State
Solid
Mintezol LogP
1.308
Mintezol Dosage Forms
400 mg and 600 mg tablets for oral administration
Mintezol Indication
For the treatment of documented ventricular arrhythmias, such as sustained ventricular tachycardia, that, in the judgment of the physician, are life-threatening.
Mintezol Pharmacology
Tocainide is a primary amine analog of lidocaine with antiarrhythmic properties useful in the treatment of ventricular arrhythmias. Tocainide, like lidocaine, produces dose dependent decreases in sodium and potassium conductance, thereby decreasing the excitability of myocardial cells. In experimental animal models, the dose-related depression of sodium current is more pronounced in ischemic tissue than in normal tissue. Tocainide is a Class I antiarrhythmic compound with electrophysiologic properties in man similar to those of lidocaine, but dissimilar from quinidine, procainamide, and disopyramide.
Mintezol Absorption
Following oral administration, the bioavailability approaches 100 percent, and is unaffected by food.
Mintezol side effects and Toxicity
The oral LD50 of tocainide was calculated to be about 800 mg/kg in mice, 1000 mg/kg in rats, and 230 mg/kg in guinea pigs; deaths were usually preceded by convulsions.
Mintezol Patient Information
Mintezol Organisms Affected
Humans and other mammals