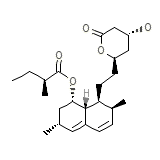
Mevinacor




Mevinacor Brand names, Mevinacor Analogs
- 6 alpha-Methylcompactin
- Altocor
- Altoprev
- Artein
- Belvas
- Cholestra
- Closterol
- Colevix
- Compactin
- Hipolip
- Hipovastin
- Lestatin
- Lipdip
- Lipivas
- Lipofren
- Lovalip
- Lovalord
- Lovastatin [Usan:Ban:Inn]
- Lovastatina [Spanish]
- Lovastatine [French]
- Lovastatinum [Latin]
- Lovasterol
- Lovastin
- Lozutin
- Mevacor
- Mevastatin
- Mevinacor
- Mevinolin
- Mevlor
- Monacolin K
- Nergadan
- Paschol
- Pravastatin
- Rodatin
- Rovacor
- Sivlor
- Taucor
- Tecnolip
- Teroltrat
Mevinacor Brand Names Mixture
- No information avaliable
Mevinacor Chemical_Formula
C24H36O5
Mevinacor RX_link
http://www.rxlist.com/cgi/generic3/altocor.htm
Mevinacor fda sheet
Mevinacor msds (material safety sheet)
Mevinacor Synthesis Reference
R. L. Monaghan et al., U.S. Pat. 4,231,938 (1980)
Mevinacor Molecular Weight
404.54 g/mol
Mevinacor Melting Point
174.5 oC
Mevinacor H2O Solubility
0.0004 mg/mL
Mevinacor State
Solid
Mevinacor LogP
4
Mevinacor Dosage Forms
Tablet
Mevinacor Indication
For management as an adjunct to diet to reduce elevated total-C, LDL-C, apo B, and TG levels in patients with primary hypercholesterolemia and mixed dyslipidemia; For primary prevention of coronary heart disease
Mevinacor Pharmacology
Lovastatin, an antilipemic agent produced by fermentation of Aspergillus terreus, is the first of a class of lipid-lowering agents known as the HMG-CoA reductase inhibitors. Lovastatin is used to treat hypercholesterolemia, to slow coronary atherosclerosis, and to prevent myocardial infarction and stroke. Lovastatin, like simvastin and unlike pravastatin, is a prodrug, concentrating active drug in the liver during first-pass circulation.
Mevinacor Absorption
30%
Mevinacor side effects and Toxicity
LD50>1000 mg/kg (orally in mice)
Mevinacor Patient Information
PATIENT INFORMATION
Lovastatin is used to lower high cholesterol levels. This medication should
not be taken if you are pregnant, nursing, or have liver disease. Lovastatin
should be taken with the evening meal. This medication may cause increased
sensitivity to sunlight. Use sunscreens and wear protective clothing until
degree of sensitivity is determined. Notify your physician if you develop
blurred vision; skin rash; muscle pain, weakness, or cramps; unexplained fever,
or yellowing of the skin or eyes.
Patients should be advised about substances they should not take concomitantly
with lovastatin and be advised to report promptly unexplained muscle pain,
tenderness, or weakness (see list below and WARNINGS, Myopathy/Rhabdomyolysis ).
Patients should also be advised to inform other physicians prescribing a new
medication that they are taking MEVACOR .
Lovastatin is used to lower high cholesterol levels. This medication should
not be taken if you are pregnant, nursing, or have liver disease. Lovastatin
should be taken with the evening meal. This medication may cause increased
sensitivity to sunlight. Use sunscreens and wear protective clothing until
degree of sensitivity is determined. Notify your physician if you develop
blurred vision; skin rash; muscle pain, weakness, or cramps; unexplained fever,
or yellowing of the skin or eyes.
Patients should be advised about substances they should not take concomitantly
with lovastatin and be advised to report promptly unexplained muscle pain,
tenderness, or weakness (see list below and WARNINGS, Myopathy/Rhabdomyolysis ).
Patients should also be advised to inform other physicians prescribing a new
medication that they are taking MEVACOR .
Mevinacor Organisms Affected
Humans and other mammals