Metadate CD




Metadate CD Brand names, Metadate CD Analogs
- 4311/B Ciba
- Calocain
- Centedein
- Centedrin
- Centedrine
- Centredin
- Concerta
- Focalin
- Focalin XR
- Methylphen
- Meridil
- Metadate
- Metadate CD
- Metadate ER
- Methyl phenidyl acetate
- Methylin
- Methylin ER
- Methylofenidan
- Methylphenidan
- Methylphenidate HCl
- Methylphenidate hydrochloride
- Methylphenidatum [Inn-Latin]
- Methylphenidylacetate hydrochloride
- Methypatch
- Metilfenidat hydrochloride
- Metilfenidato [Inn-Spanish]
- Metilfenidato [Italian]
- Phenidylate
- Plimasine
- PMS-Methylphenidate
- Riphenidate
- Ritalin
- Ritalin hydrochloride
- Ritalin LA
- Ritalin SR
- Ritalin-SR
- Ritaline
- Ritcher Works
Metadate CD Brand Names Mixture
- No information avaliable
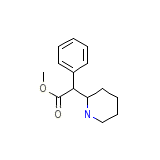
Metadate CD Chemical_Formula
C14H19NO2
Metadate CD RX_link
http://www.rxlist.com/cgi/generic/methphen.htm
Metadate CD fda sheet
Metadate CD msds (material safety sheet)
Metadate CD Synthesis Reference
M. Hartmann, L. Panizzon, U.S. Pat. 2,507,631 (1950)
Metadate CD Molecular Weight
233.306 g/mol
Metadate CD Melting Point
224-226oC
Metadate CD H2O Solubility
1255mg/L
Metadate CD State
Solid
Metadate CD LogP
3.192
Metadate CD Dosage Forms
Tablets; Tablets (sustained release)
Metadate CD Indication
For use as an integral part of a total treatment program which typically includes other remedial measures (psychological, educational, social) for a stabilizing effect in children with a behavioral syndrome characterized by the following group of developmentally inappropriate symptoms: moderate-to-severe distractibility, short attention span, hyperactivity, emotional lability, and impulsivity.
Metadate CD Pharmacology
Methylphenidate is a central nervous system stimulant used most commonly in the treatment of attention-deficit disorders in children and for narcolepsy. Its mechanisms appear to be similar to those of dextroamphetamine.
Metadate CD Absorption
Readily absorbed in a biphasic manner. It reaches peak absorption at approximately two hours for the first phase and five hours for the second phase. Bioavailability is low (approximately 30%)
Metadate CD side effects and Toxicity
Symptoms of overdose include vomiting, agitation, tremors, hyperreflexia, muscle twitching, convulsions (may be followed by coma), euphoria, confusion, hallucinations, delirium, sweating, flushing, headache, hyperpyrexia, tachycardia, palpitations, cardiac arrhythmias, hypertension, mydriasis, and dryness of mucous membranes. LD50=190mg/kg (orally in mice)
Metadate CD Patient Information
Metadate CD Organisms Affected
Humans and other mammals