Meiact




Meiact Brand names, Meiact Analogs
Meiact Brand Names Mixture
- No information avaliable
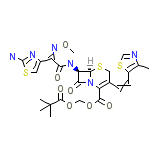
Meiact Chemical_Formula
C25H28N6O7S3
Meiact RX_link
http://www.rxlist.com/cgi/generic3/spectracef.htm
Meiact fda sheet
Meiact msds (material safety sheet)
Meiact Synthesis Reference
No information avaliable
Meiact Molecular Weight
620.724 g/mol
Meiact Melting Point
127 - 129 oC
Meiact H2O Solubility
Soluble at levels equal to < 0.1 mg/mL.
Meiact State
Solid
Meiact LogP
2.721
Meiact Dosage Forms
Tablets (200mg) for oral administration.
Meiact Indication
For the treatment of mild to moderate infections in adults and adolescents (12 years of age or older) which are caused by susceptible strains of microorganisms in acute bacterial exacerbation of chronic bronchitis, community-acquired pneumonia, pharyngitis/tonsillitis, and uncomplicated skin and skin-structure infections.
Meiact Pharmacology
Cefditoren pivoxil is a prodrug which is hydrolyzed by esterases during absorption, and the drug is distributed in the circulating blood as active cefditoren. Cefditoren is a cephalosporin with antibacterial activity against gram-positive and gram-negative pathogens. Cefditoren is effective against Staphylococcus aureus (methicillin-susceptible strains, including b-lactamase-producing strains), penicillin-susceptible strains of Staphylococcus aureus and Streptococcus pneumoniae, Streptococcus pyogenes, Haemophilus influenzae (including b-lactamase-producing strains), Haemophilus parainfluenzae (including b-lactamase-producing strains), Moraxella catarrhalis (including b-lactamase-producing strains), Streptococcus agalactiae, Streptococcus Groups C and G, and Streptococcus, viridans group (penicillin-susceptible and -intermediate strains).
Meiact Absorption
Following oral administration, cefditoren pivoxil is absorbed from the gastrointestinal tract and hydrolyzed to cefditoren by esterases. Under fasting conditions, the estimated absolute bioavailability of cefditoren pivoxil is approximately 14%. The absolute bioavailability of cefditoren pivoxil administered with a low fat meal (693 cal, 14 g fat, 122 g carb, 23 g protein) is 16.1 ± 3.0%.
Meiact side effects and Toxicity
Information on cefditoren pivoxil overdosage in humans is not available. However, with other b-lactam antibiotics, adverse effects following overdosage have included nausea, vomiting, epigastric distress, diarrhea, and convulsions. In acute animal toxicity studies, cefditoren pivoxil when tested at the limit oral doses of 5100 mg/kg in rats and up to 2000 mg/kg in dogs did not exhibit any health effects of concern.
Meiact Patient Information
No information avaliable
Meiact Organisms Affected
Enteric bacteria and other eubacteria