Megeron




Megeron Brand names, Megeron Analogs
Megeron Brand Names Mixture
- No information avaliable
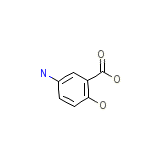
Megeron Chemical_Formula
C7H7NO3
Megeron RX_link
http://www.rxlist.com/cgi/generic2/canasa.htm
Megeron fda sheet
Megeron msds (material safety sheet)
Megeron Synthesis Reference
No information avaliable
Megeron Molecular Weight
153.135 g/mol
Megeron Melting Point
283 oC
Megeron H2O Solubility
0.84 g/L at 20°C
Megeron State
Solid
Megeron LogP
1.012
Megeron Dosage Forms
Rectal suppository (500 mg or 1000 mg)
Megeron Indication
For the treatment of active ulcerative proctitis.
Megeron Pharmacology
Sulfasalazine has been used in the treatment of ulcerative colitis for over 55 years. It is split by bacterial action in the colon into sulfapyridine (SP) and mesalamine (5-ASA). It is thought that the mesalamine component only is therapeutically active in ulcerative colitis.
Megeron Absorption
20 to 30% absorbed following oral administration. 10 to 35% absorbed from the colon (rectal suppository) - extent of absorption is determined by the length of time the drug is retained in the colon.
Megeron side effects and Toxicity
Oral, mouse: LD50 = 3370 mg/kg; Oral, rat: LD50 = 2800 mg/kg; Skin, rabbit: LD50 = >5 gm/kg. There have been no documented reports of serious toxicity in man resulting from massive overdosing with mesalamine. Under ordinary circumstances, mesalamine absorption from the colon is limited.
Megeron Patient Information
Megeron Organisms Affected
Humans and other mammals