Maxalt-MLT




Maxalt-MLT Brand names, Maxalt-MLT Analogs
Maxalt-MLT Brand Names Mixture
- No information avaliable
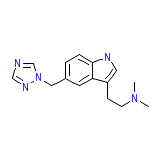
Maxalt-MLT Chemical_Formula
C15H19N5
Maxalt-MLT RX_link
http://www.rxlist.com/cgi/generic2/rizatrip.htm
Maxalt-MLT fda sheet
Maxalt-MLT msds (material safety sheet)
Maxalt-MLT Synthesis Reference
No information avaliable
Maxalt-MLT Molecular Weight
269.345 g/mol
Maxalt-MLT Melting Point
178-180 oC
Maxalt-MLT H2O Solubility
42 mg/mL (for free base)
Maxalt-MLT State
Solid
Maxalt-MLT LogP
2.129
Maxalt-MLT Dosage Forms
Tablet; Wafer
Maxalt-MLT Indication
For treatment of acute migraine attacks.
Maxalt-MLT Pharmacology
Rizatriptan is a selective 5-hydroxytryptamine receptor subtype agonist indicated for the acute treatment of migraine attacks with or without aura in adults. Rizatriptan is not intended for the prophylactic therapy of migraine or for use in the management of hemiplegic or basilar migraine. Rizatriptan is an agonist for a vascular 5-hydroxytryptamine receptor subtype (probably a member of the 5-HT1D family) having only a weak affinity for 5-HT1A, 5-HT5A, and 5-HT7 receptors and no significant affinity or pharmacological activity at 5-HT2, 5-HT3 or 5-HT4 receptor subtypes or at alpha1-, alpha2-, or beta-adrenergic, dopamine1,; dopamine2; muscarinic, or benzodiazepine receptors. This action in humans correlates with the relief of migraine headache. In addition to causing vasoconstriction, experimental data from animal studies show that Rizatriptan also activates 5-HT1 receptors on peripheral terminals of the trigeminal nerve innervating cranial blood vessels, which may also contribute to the antimigrainous effect of Rizatriptan in humans.
Maxalt-MLT Absorption
Rapid following oral administration. Bioavailability is 45%. Food has no effect on the bioavailability of rizatriptan. However, administering rizatriptan with food will delay by 1 hour the time to reach peak plasma concentration. The rate of absorption is not affected by the presence of a migraine attack.
Maxalt-MLT side effects and Toxicity
Symptoms of overdose include dizziness, fainting, heart and blood vessel problems, high blood pressure, loss of bowel and bladder control, slow heartbeat, and vomiting.
Maxalt-MLT Patient Information
Migraine or treatment with MAXALT may cause somnolence in some patients. Dizziness has also been reported in some patients receiving MAXALT. Patients should, therefore, evaluate their ability to perform complex tasks during migraine attacks and after administration of MAXALT. Physicians should instruct their patients to read the patient package insert before taking MAXALT.
Maxalt-MLT Organisms Affected
Humans and other mammals