Livostin




Livostin Brand names, Livostin Analogs
Livostin Brand Names Mixture
- No information avaliable
Livostin Chemical_Formula
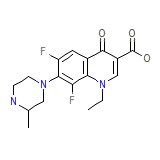
C17H19F2N3O3
Livostin RX_link
http://www.rxlist.com/cgi/generic2/lomefloxacin.htm
Livostin fda sheet
Livostin msds (material safety sheet)
Livostin Synthesis Reference
No information avaliable
Livostin Molecular Weight
351.348 g/mol
Livostin Melting Point
239-240.5 oC
Livostin H2O Solubility
27.2 mg/mL
Livostin State
Solid
Livostin LogP
1.781
Livostin Dosage Forms
Tablet
Livostin Indication
For the treatment of bacterial infections of the respiratory tract (chronic bronchitis) and urinary tract, and as a pre-operative prophylactic to prevent urinary tract infection caused by: S.pneumoniae, H.influenzae, S.aureus, P.aeruginosa, E. cloacae, P. mirabilis, C. civersus, S. asprphyticus, E.coli, and K.pneumoniae.
Livostin Pharmacology
Lomefloxacin is a fluoroquinolone antibiotic used to treat chronic bronchitis, as well as complicated and uncomplicated urinary tract infections. It is also used as a prophylactic or preventative treatment to prevent urinary tract infections in patients undergoing transrectal or transurethral surgical procedures. Flouroquinolones such as lomefloxacin possess excellent activity against gram-negative aerobic bacteria such as E.coli and Neisseria gonorrhoea as well as gram-positive bacteria including S. pneumoniae and Staphylococcus aureus. They also posses effective activity against shigella, salmonella, campylobacter, gonococcal organisms, and multi drug resistant pseudomonas and enterobacter.
Livostin Absorption
Rapid and nearly complete with approximately 95% to 98% of a single oral dose being absorbed.
Livostin side effects and Toxicity
Adverse reactions include peripheral neuropathy, nervousness, agitation, anxiety, and phototoxic events (rash, itching, burning) due to sunlight exposure.
Livostin Patient Information
Livostin Organisms Affected
Enteric bacteria and other eubacteria