Linezlid




Linezlid Brand names, Linezlid Analogs
Linezlid Brand Names Mixture
- Gastrografin - Liq Orl (Diatrizoate Meglumine + Diatrizoate Sodium)
- Gastrografin Liq (Diatrizoate Meglumine + Diatrizoate Sodium)
- Hypaque-M 76% (Diatrizoate Meglumine + Diatrizoate Sodium)
- Hypaque-M 76% Liq (Diatrizoate Meglumine + Diatrizoate Sodium)
- Hypaque-M Liq 76% Usp (Diatrizoate Meglumine + Diatrizoate Sodium)
- Md-60 (Diatrizoate Meglumine + Diatrizoate Sodium)
- Md-76 (Diatrizoate Meglumine + Diatrizoate Sodium)
- Renocal-76 (Diatrizoate Meglumine + Diatrizoate Sodium)
- Renografin-60 - Liq Iv (Diatrizoate Meglumine + Diatrizoate Sodium)
- Renografin-60 Liq (Diatrizoate Meglumine + Diatrizoate Sodium)
- Renografin-76 - Liq Iv (Diatrizoate Meglumine + Diatrizoate Sodium)
- Renografin-76 Liq (Diatrizoate Meglumine + Diatrizoate Sodium)
- Sinografin (Diatrizoate Meglumine + Meglumine Iodipamide)
- Sinografin - Liq Iu (Diatrizoate Meglumine + Meglumine Iodipamide)
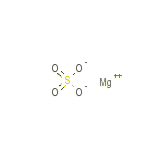
Linezlid Chemical_Formula
MgSO4
Linezlid RX_link
http://www.rxlist.com/cgi/generic3/mgso4.htm
Linezlid fda sheet
Linezlid msds (material safety sheet)
Linezlid Synthesis Reference
No information avaliable
Linezlid Molecular Weight
120.369 g/mol
Linezlid Melting Point
1124 oC (decomposition)
Linezlid H2O Solubility
710 mg/mL
Linezlid State
Solid
Linezlid LogP
-0.91
Linezlid Dosage Forms
Capsule; Drops; Liquid; Powder; Powder for solution; Solution; Tablet
Linezlid Indication
Used for immediate control of life-threatening convulsions in the treatment of severe toxemias (pre-eclampsia and eclampsia) of pregnancy and in the treatment of acute nephritis in children. Also indicated for replacement therapy in magnesium deficiency, especially in acute hypomagnesemia accompanied by signs of tetany similar to those of hypocalcemia. Also used in uterine tetany as a myometriat relaxant.
Linezlid Pharmacology
Magnesium sulfate is a small colorless crystal used as an anticonvulsant, a cathartic, and an electrolyte replenisher in the treatment of pre-eclampsia and eclampsia. It causes direct inhibition of action potentials in myometrial muscle cells. Excitation and contraction are uncoupled, which decreases the frequency and force of contractions. Magnesium sulfate is gaining popularity as an initial treatment in the management of various dysrhythmias, particularly torsades de pointes, and dyrhythmias secondary to TCA overdose or digitalis toxicity.
Linezlid Absorption
No information avaliable
Linezlid side effects and Toxicity
LD50 = 1200 mg/kg (rat, subcutaneous). May be harmful if swallowed. May act as an irritant. Adverse reactions include hypotension, ECG changes, diarrhea, urinary retention, CNS depression and respiratory depression.
Linezlid Patient Information
Linezlid Organisms Affected
Humans and other mammals