Lanvis




Lanvis Brand names, Lanvis Analogs
Lanvis Brand Names Mixture
- No information avaliable
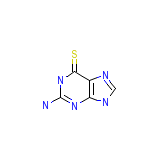
Lanvis Chemical_Formula
C5H5N5S
Lanvis RX_link
http://www.rxlist.com/cgi/generic2/thioguanine.htm
Lanvis fda sheet
Lanvis msds (material safety sheet)
Lanvis Synthesis Reference
Elion et al.; J.Amer.Chem.Soc.; 81;1898,1901 (1959)
Lanvis Molecular Weight
167.193 g/mol
Lanvis Melting Point
>360 oC
Lanvis H2O Solubility
36.3 mg/mL
Lanvis State
Solid
Lanvis LogP
0.086
Lanvis Dosage Forms
Oral tablets
Lanvis Indication
For remission induction and remission consolidation treatment of acute nonlymphocytic leukemias.
Lanvis Pharmacology
Thioguanine is an antineoplastic anti-metabolite used in the treatment of several forms of leukemia including acute nonlymphocytic leukemia. Anti-metabolites masquerade as purine or pyrimidine - which become the building blocks of DNA. They prevent these substances becoming incorporated in to DNA during the "S" phase (of the cell cycle), stopping normal development and division. Thioguanine was first synthesized and entered into clinical trial more than 30 years ago. It is a 6-thiopurine analogue of the naturally occurring purine bases hypoxanthine and guanine. Intracellular activation results in incorporation into DNA as a false purine base. An additional cytotoxic effect is related to its incorporation into RNA. Thioguanine is cross-resistant with mercaptopurine. Cytotoxicity is cell cycle phase-specific (S-phase).
Lanvis Absorption
Absorption of an oral dose is incomplete and variable, averaging approximately 30% of the administered dose (range: 14% to 46%)
Lanvis side effects and Toxicity
Oral, mouse: LD50 = 160 mg/kg. Symptoms of overdose include nausea, vomiting, malaise, hypotension, and diaphoresis.
Lanvis Patient Information
PATIENT INFORMATION
Patients should be informed that the major toxicities of thioguanine are related to
myelosuppression, hepatotoxicity, and gastrointestinal toxicity. Patients should never
be allowed to take the drug without medical supervision and should be advised to consult
their physician if they experience fever, sore throat, jaundice, nausea, vomiting, signs
of local infection, bleeding from any site, or symptoms suggestive of anemia. Women of
childbearing potential should be advised to avoid becoming pregnant.
Patients should be informed that the major toxicities of thioguanine are related to
myelosuppression, hepatotoxicity, and gastrointestinal toxicity. Patients should never
be allowed to take the drug without medical supervision and should be advised to consult
their physician if they experience fever, sore throat, jaundice, nausea, vomiting, signs
of local infection, bleeding from any site, or symptoms suggestive of anemia. Women of
childbearing potential should be advised to avoid becoming pregnant.
Lanvis Organisms Affected
Humans and other mammals