ITR




ITR Brand names, ITR Analogs
ITR Brand Names Mixture
- No information avaliable
ITR Chemical_Formula
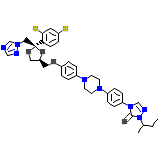
C35H38Cl2N8O4
ITR RX_link
http://www.rxlist.com/cgi/generic/itraconazole.htm
ITR fda sheet
ITR msds (material safety sheet)
ITR Synthesis Reference
No information avaliable
ITR Molecular Weight
705.633 g/mol
ITR Melting Point
166.2 oC
ITR H2O Solubility
Insoluble
ITR State
Solid
ITR LogP
6.939
ITR Dosage Forms
Capsules (100 mg); Liquid solution
ITR Indication
For the treatment of the following fungal infections in immunocompromised and non-immunocompromised patients: pulmonary and extrapulmonary blastomycosis, histoplasmosis, aspergillosis, and onychomycosis.
ITR Pharmacology
Itraconazole is an imidazole/triazole type antifungal agent. Itraconazole is a highly selective inhibitor of fungal cytochrome P-450 sterol C-14 α-demethylation via the inhibition of the enzyme cytochrome P450 14α-demethylase. This enzyme converts lanosterol to ergosterol, and is required in fungal cell wall synthesis. The subsequent loss of normal sterols correlates with the accumulation of 14 α-methyl sterols in fungi and may be partly responsible for the fungistatic activity of fluconazole. Mammalian cell demethylation is much less sensitive to fluconazole inhibition. Itraconazole exhibits in vitro activity against Cryptococcus neoformans and Candida spp. Fungistatic activity has also been demonstrated in normal and immunocompromised animal models for systemic and intracranial fungal infections due to Cryptococcus neoformans and for systemic infections due to Candida albicans.
ITR Absorption
The absolute oral bioavailability of itraconazole is 55%, and is maximal when taken with a full meal.
ITR side effects and Toxicity
No significant lethality was observed when itraconazole was administered orally to mice and rats at dosage levels of 320 mg/kg or to dogs at 200 mg/kg.
ITR Patient Information
No information avaliable
ITR Organisms Affected
Fungi, yeast and protozoans