Hydergin




Hydergin Brand names, Hydergin Analogs
- Alkergot
- Circanol
- Co-Dergocrine Mesylate
- Deapril-ST
- Dihydroergotoxin Mesilat
- Dihydroergotoxin Mesylate
- Dihydroergotoxin Methanesulfonate
- Dihydroergotoxine Mesilate
- Dihydroergotoxine Mesylate
- Dihydroergotoxine Methanesulfonate
- Dihydroergotoxine Methanesulphonate
- Ergoloid Mesylates [Usan]
- Gerimal
- Hydergin
- Hydergine
- Hydergine LC
- Hydrogenated Ergot Alkaloids
- Ischelium
- Redergin
- Trigot
Hydergin Brand Names Mixture
- No information avaliable
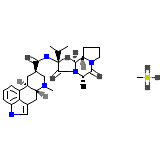
Hydergin Chemical_Formula
C33H45N5O5
Hydergin RX_link
No information avaliable
Hydergin fda sheet
Hydergin msds (material safety sheet)
Hydergin Synthesis Reference
No information avaliable
Hydergin Molecular Weight
591.741 g/mol
Hydergin Melting Point
No information avaliable
Hydergin H2O Solubility
No information avaliable
Hydergin State
Solid
Hydergin LogP
2.615
Hydergin Dosage Forms
Capsule; Liquid; Powder; Powder for solution; Solution; Syrup; Tablet
Hydergin Indication
For use as an adjunct therapy for patients with dementia
Hydergin Pharmacology
Ergoloid Mesylate may increase cerebral metabolism and blood flow. The role of this medication in the therapy of dementia is controversial. A recent controlled study in patients with Alzheimer's disease found that there was no advantage to the use of ergoloid mesylates compared to placebo, suggesting that ergoloid mesylates may lower scores on some cognitive and behavioral rating scales. Further study is needed to determine the risk-benefit profile of ergoloid mesylates in the treatment of dementia.
Hydergin Absorption
Rapidly but incompletely (approximately 25%) absorbed from the gastrointestinal tract. Approximately 50% of the absorbed dose is eliminated by first-pass metabolism.
Hydergin side effects and Toxicity
Symptoms of overdose include dyspnea, hypotension or hypertension, rapid weak pulse, delirium, nausea, vomiting, and bradycardia.
Hydergin Patient Information
No information avaliable
Hydergin Organisms Affected
Humans and other mammals