Ganirelix Acetate




Ganirelix Acetate Brand names, Ganirelix Acetate Analogs
Ganirelix Acetate Brand Names Mixture
- No information avaliable
Ganirelix Acetate Chemical_Formula
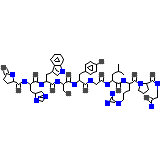
C55H75N17O13
Ganirelix Acetate RX_link
http://www.rxlist.com/cgi/generic2/gonadorelin.htm
Ganirelix Acetate fda sheet
Ganirelix Acetate msds (material safety sheet)
Ganirelix Acetate Synthesis Reference
No information avaliable
Ganirelix Acetate Molecular Weight
1182.29 g/mol
Ganirelix Acetate Melting Point
No information avaliable
Ganirelix Acetate H2O Solubility
1 mg/mL
Ganirelix Acetate State
Liquid
Ganirelix Acetate LogP
-4.634
Ganirelix Acetate Dosage Forms
Liquid; Powder for solution; Solution for infusion pump
Ganirelix Acetate Indication
For evaluating the functional capacity and response of the gonadotropes of the anterior pituitary also for evaluating residual gonadotropic function of the pituitary following removal of a pituitary tumor by surgery and/or irradiation.
Ganirelix Acetate Pharmacology
Gonadorelin is responsible for the release of follicle stimulating hormone and leutinizing hormone from the anterior pitutitary. In the pituitary GnRH stimulates synthesis and release of FSH and LH, a process that is controlled by the frequency and amplitude of GnRH pulses, as well as the feedback of androgens and estrogens. The pulsatility of GnRH secretion has been seen in all vertebrates, and it is necessary to ensure a correct reproductive function. Thus a single hormone, GnRH, controls a complex process of follicular growth, ovulation, and corpus luteum maintenance in the female, and spermatogenesis in the male. Its short half life requires infusion pumps for its clinical use
Ganirelix Acetate Absorption
Rapidly absorbed when injected
Ganirelix Acetate side effects and Toxicity
LD50>3000 mg/kg (rat, oral)
Ganirelix Acetate Patient Information
Ganirelix Acetate Organisms Affected
Humans and other mammals