Gabbropas




Gabbropas Brand names, Gabbropas Analogs
- Aminosalicylate Sodium
- Amino-Pas
- Aminopar
- Aminox
- Apacil
- Apas
- Deapasil
- Entepas
- Ferrosan
- Gabbropas
- Hellipidyl
- Kyselina P-Aminosalicylova
- Neopasalate
- Osacyl
- P-Aminosalicylic Acid
- PAS
- PAS-C
- PASK
- Pamacyl
- Pamisyl
- Para-Amino Salicylic Acid
- Para-Pas
- Paramycin
- Parasal
- Parasalicil
- Parasalindon
- Pasa
- Pasalon
- Pasara
- Pascorbic
- Pasdium
- Pasem
- Paser
- Pasmed
- Pasnodia
- Pasolac
- Propasa
- Rezipas
- Sanipirol-4
- Sanipriol-4
Gabbropas Brand Names Mixture
- No information avaliable
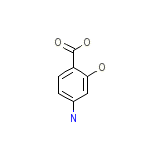
Gabbropas Chemical_Formula
C7H7NO3
Gabbropas RX_link
http://www.rxlist.com/cgi/generic2/paser.htm
Gabbropas fda sheet
Gabbropas msds (material safety sheet)
Gabbropas Synthesis Reference
Biniecki S., et al. Acta Pol Pharm. 1967;24(5):469-73
Gabbropas Molecular Weight
153.135 g/mol
Gabbropas Melting Point
150.5 oC
Gabbropas H2O Solubility
1690 mg/L
Gabbropas State
Solid
Gabbropas LogP
1.012
Gabbropas Dosage Forms
Packet of coated granules
Gabbropas Indication
For the treatment of tuberculosis
Gabbropas Pharmacology
Aminosalicylic acid is an anti-mycobacterial agent used with other anti-tuberculosis drugs (most often isoniazid) for the treatment of all forms of active tuberculosis due to susceptible strains of tubercle bacilli. The two major considerations in the clinical pharmacology of aminosalicylic acid are the prompt production of a toxic inactive metabolite under acid conditions and the short serum half life of one hour for the free drug. Aminosalicylic acid is bacteriostatic against Mycobacterium tuberculosis (prevents the multiplying of bacteria without destroying them). It also inhibits the onset of bacterial resistance to streptomycin and isoniazid.
Gabbropas Absorption
No information avaliable
Gabbropas side effects and Toxicity
LD50=4 gm/kg (orally in mice); LD50=3650 mg/kg (orally in rabbits)
Gabbropas Patient Information
Gabbropas Organisms Affected
Mycobacteria