Flumadine




Flumadine Brand names, Flumadine Analogs
Flumadine Brand Names Mixture
- No information avaliable
Flumadine Chemical_Formula
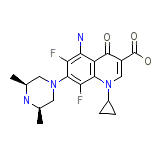
C19H22F2N4O3
Flumadine RX_link
http://www.rxlist.com/cgi/generic2/sparfloxacin.htm
Flumadine fda sheet
Flumadine msds (material safety sheet)
Flumadine Synthesis Reference
No information avaliable
Flumadine Molecular Weight
392.4 g/mol
Flumadine Melting Point
No information avaliable
Flumadine H2O Solubility
Practically insoluble
Flumadine State
Solid
Flumadine LogP
1.695
Flumadine Dosage Forms
Tablets (200-mg round, white, film-coated)
Flumadine Indication
For the treatment of adults with the following infections caused by susceptible strains microorganisms: community-acquired pneumonia (caused by Chlamydia pneumoniae, Haemophilus influenzae, Haemophilus parainfluenzae, Moraxella catarrhalis, Mycoplasma pneumoniae, or Streptococcus pneumoniae) and acute bacterial exacerbations of chronic bronchitis (caused by Chlamydia pneumoniae, Enterobacter cloacae, Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Staphylococcus aureus, or Streptococcus pneumoniae).
Flumadine Pharmacology
Sparfloxacin is a synthetic fluoroquinolone broad-spectrum antimicrobial agent in the same class as ofloxacin and norfloxacin. Sparfloxacin has in vitro activity against a wide range of gram-negative and gram-positive microorganisms. Sparfloxacin exerts its antibacterial activity by inhibiting DNA gyrase, a bacterial topoisomerase. DNA gyrase is an essential enzyme which controls DNA topology and assists in DNA replication, repair, deactivation, and transcription. Quinolones differ in chemical structure and mode of action from (beta)-lactam antibiotics. Quinolones may, therefore, be active against bacteria resistant to (beta)-lactam antibiotics. Although cross-resistance has been observed between sparfloxacin and other fluoroquinolones, some microorganisms resistant to other fluoroquinolones may be susceptible to sparfloxacin. In vitro tests show that the combination of sparfloxacin and rifampin is antagonistic against Staphylococcus aureus.
Flumadine Absorption
Well absorbed following oral administration with an absolute oral bioavailability of 92%. Unaffected by administration with milk or food, however concurrent administration of antacids containing magnesium hydroxide and aluminum hydroxide reduces the oral bioavailability of sparfloxacin by as much as 50%.
Flumadine side effects and Toxicity
Single doses of sparfloxacin were relatively non-toxic via the oral route of administration in mice, rats, and dogs. No deaths occurred within a 14-day post-treatment observation period at the highest oral doses tested, up to 5000 mg/kg in either rodent species, or up to 600 mg/kg in the dog. Clinical signs observed included inactivity in mice and dogs, diarrhea in both rodent species, and vomiting, salivation, and tremors in dogs.
Flumadine Patient Information
Flumadine Organisms Affected
Enteric bacteria and other eubacteria