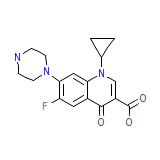
Flociprin




Flociprin Brand names, Flociprin Analogs
- Bacquinor
- Baycip
- Bernoflox
- Ciprofloxacin HCl
- Ciflox
- Cifloxin
- Ciloxan
- Ciprinol
- Cipro
- Cipro I.V.
- Cipro XL
- Cipro XR
- Ciprobay
- Ciprocinol
- Ciprodar
- Ciprofloxacin Base
- Ciprofloxacin dihydrochloride
- Ciprofloxacin hydrochloride
- Ciprofloxacin monohydrochloride
- Ciprofloxacina
- Cipromycin
- Ciproquinol
- Ciproxan
- Ciproxin
- Flociprin
- Floxin
- Floxin Otic
- Ocuflox
- Ofloxacin
- Proquin XR
- Septicide
- Velomonit
Flociprin Brand Names Mixture
- Cipro HC Otic Suspension (Ciprofloxacin hydrochloride + Hydrocortisone)
- Ciprodex (Ciprofloxacin hydrochloride + Dexamethasone)
Flociprin Chemical_Formula
Flociprin RX_link
Flociprin fda sheet
Flociprin msds (material safety sheet)
Flociprin Synthesis Reference
Flociprin Molecular Weight
Flociprin Melting Point
Flociprin H2O Solubility
Flociprin State
Flociprin LogP
Flociprin Dosage Forms
Flociprin Indication
Flociprin Pharmacology
Flociprin Absorption
Flociprin side effects and Toxicity
Flociprin Patient Information
Patients should be advised:
· that antibacterial drugs, including Proquin XR, should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Proquin XR is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Proquin XR or other antibacterial drugs in the future.
· that Proquin XR should only be used to treat uncomplicated urinary tract infections (also known as bladder infections). The safety and efficacy of Proquin XR to treat other urinary tract or non-urinary tract infections have not been studied.
· that Proquin XR should be taken with a main meal of the day, preferably the evening meal. The patient should not take more than one Proquin XR tablet per day, even if the patient misses a dose.
· that Proquin XR tablets should be taken whole and never split, crushed, or chewed.
· that concomitant administration of Proquin XR with aluminum or magnesium-containing antacids, sucralfate, VIDEX (didanosine) chewable buffered tablets or pediatric powder, metal cations such as iron and calcium, and multivitamin preparations containing zinc should be avoided. Proquin XR should be administered at least 4 hours before or 2 hours after these products.
· that Proquin XR should not be taken with dairy products (like milk or yogurt) or calcium-fortified juices alone, since the absorption of ciprofloxacin may be significantly reduced. However, Proquin XR may be taken with a meal that contains these products.
· that ciprofloxacin may be associated with hypersensitivity reactions, even following a single dose, and to discontinue Proquin XR at the first sign of a skin rash or other allergic reaction and contact their physician.
· to avoid excessive sunlight or artificial ultraviolet (UV) light while receiving Proquin XR and to discontinue therapy if phototoxicity occurs.
· that peripheral neuropathies have been associated with ciprofloxacin use. If symptoms of peripheral neuropathy including pain, burning, tingling, numbness and/or weakness develop, patients should discontinue treatment and contact their physician.
· that if they experience pain, inflammation, or rupture of a tendon to discontinue treatment, to inform their physician, and to rest and refrain from exercise.
· to contact their doctor if they do not feel better of if they develop fever and back pain while or after taking Proquin XR.
· that Proquin XR may cause dizziness and lightheadedness; therefore, patients should know how they react to this drug before they operate an automobile or machinery or engage in activities requiring mental alertness or coordination.
· that Proquin XR may increase the effects of theophylline and caffeine. There is a possibility of caffeine accumulation when products containing caffeine are consumed while taking quinolones.
· that convulsions have been reported in patients receiving quinolones, including ciprofloxacin, and to notify their physician before taking this drug if there is a history of this condition.
Patient Leaflet
PROQUIN XR (ciprofloxacin hydrochloride) Extended-Release Tablets
PROQUIN® XR
(prōkwin)
(ciprofloxacin hydrochloride)
Extended-Release Tablets, 500 mg
This leaflet contains important information about Proquin XR (ciprofloxacin hydrochloride) extended-release tablets and should be read before you begin treatment. This leaflet does not replace talking with your doctor about your medical condition or your treatment. This leaflet does not list all benefits and risks of Proquin XR. Proquin XR can be prescribed only by a doctor. If you have any questions about Proquin XR, talk to your doctor. Only your doctor can tell you if Proquin XR is right for you.
What is Proquin XR?
Proquin XR is an antibiotic in the class known as "quinolones" that is used to treat adults with simple (uncomplicated) urinary tract infections (also known as "bladder infections") caused by bacteria. It is not known if Proquin XR will treat infections other than bladder infections. Proquin XR, like all other antibiotics, does not kill viruses.
You should contact your doctor if you do not feel better or if you develop fever and back pain while or after taking Proquin XR.
Proquin XR tablets are blue and contain 500 mg of active drug.
How should I take Proquin XR?
· Proquin XR should be taken once a day for 3 days shortly after a main meal of the day, preferably the evening meal. Proquin XR does not work as well if you take it without a meal. You should try to take Proquin XR at about the same time each day.
· Take Proquin XR for all 3 days, even if you are feeling better. If you stop taking Proquin XR before all 3 doses, Proquin XR may not cure your bladder infection.
· Do not split, crush, or chew Proquin XR tablets. Proquin XR tablets must be swallowed whole. Tell your doctor if you cannot swallow tablets whole. Your doctor will prescribe a different medicine for you.
· Do not take more than one Proquin XR tablet a day, even if you miss a dose.
· Do not take Proquin XR at the same time that you drink milk or juices with added calcium, unless you drink them with a main meal.
· Many antacids and multivitamins may interfere with the absorption of Proquin XR if taken at the same time. Take Proquin XR at least 4 hours before or 2 hours after antacids that contain magnesium or aluminum. Proquin XR should also be taken at least 4 hours before or 2 hours after sucralfate, VIDEX® (didanosine) chewable buffered tablets or pediatric powder, iron, calcium, and vitamins that contain zinc.
Who should not take Proquin XR?
Do not take Proquin XR if you are allergic to or have ever had a severe reaction to ciprofloxacin or to any other "quinolone" antibiotics.
Proquin XR is not recommended for use during pregnancy or nursing, as the effects on the unborn child or nursing infant are unknown. If you are pregnant or planning to become pregnant while taking Proquin XR, talk to your doctor before taking this medication.
Proquin XR is not recommended for children.
What should I tell my doctor before taking Proquin XR?
Tell you doctor about all of your medical conditions, including if you have or ever had seizures (epilepsy), asthma, or liver or kidney problems.
Tell your doctor about all the medicines you take, including prescription and nonprescription medicines, vitamins and herbal supplements. Proquin XR and certain other medicines can affect each other. You may have to adjust the times you take certain other medicines, vitamins, and herbal supplements. Especially, tell your doctor if you take: theophylline, VIDEX® (didanosine) chewable buffered tablets or pediatric powder; warfarin (Coumadin®); glyburide (Glucovance®, Micronase®, DiaBeta®); phenytoin (Dilantin®); sucralfate (Carafate®); or antacids or vitamins that contain magnesium, calcium, aluminum, iron, or zinc.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist.
What are the possible side effects of Proquin XR?
Proquin XR is generally well tolerated. The most common side effects with Proquin XR include vaginal yeast infection and headache. Less common side effects include nausea, diarrhea, dizziness, and abdominal pain.
You should be careful about driving or operating machinery until you are sure the Proquin XR is not causing dizziness or lightheadedness.
Rare cases of allergic reactions have been reported in patients receiving quinolones, including ciprofloxacin, even after just one dose. Stop taking Proquin XR and call your doctor or get emergency medical attention right away if you develop a rash, hives, swelling of your face or throat, or have trouble breathing.
Some patients taking quinolone antibiotics may become more sensitive to sunlight or ultraviolet light such as that used in tanning salons. You should avoid excessive exposure to sunlight or ultraviolet light while taking Proquin XR.
Ciprofloxacin has rarely been associated with inflammation of the tendons. Stop taking Proquin XR and call your doctor if you experience pain, swelling, or rupture of a tendon.
Convulsions have been reported in patients receiving quinolone antibiotics including ciprofloxacin. Tell your doctor if you have experienced convulsions in the past. Quinolones, including ciprofloxacin, have been rarely associated with other central nervous system events including confusion, tremors, hallucinations, and depression. Stop taking Proquin XR and call your doctor right away if you get any of these symptoms.
These are not all the side effects with Proquin XR. For more information, ask your doctor or pharmacist.
How should I store Proquin XR?
· Store Proquin XR at room temperature, 59° to 86° F (15° to 30° C).
· Keep Proquin XR and all medicines out of the reach of children.
General information about Proquin XR
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use Proquin XR for a condition for which it was not prescribed. Do not give Proquin XR to other people, even if they have the same symptoms you have. It may harm them.
Keep this medication out of the reach of children.
This leaflet summarizes the most important information about Proquin XR. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about Proquin XR that is written for health care professionals. Further information is also provided at:
1-800-206-2945 and www.Proquin.com
What are the ingredients in Proquin XR?
Active Ingredient: ciprofloxacin hydrochloride
Inactive Ingredients: film coating, magnesium stearate, polyethylene oxide, and povidone