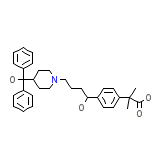
Fexofendine




Fexofendine Brand names, Fexofendine Analogs
Fexofendine Brand Names Mixture
- Allegra-D (Fexofenadine hydrochloride + Pseudoephedrine hydrochloride)
Fexofendine Chemical_Formula
Fexofendine RX_link
Fexofendine fda sheet
Fexofendine msds (material safety sheet)
Fexofendine Synthesis Reference
Fexofendine Molecular Weight
Fexofendine Melting Point
Fexofendine H2O Solubility
Fexofendine State
Fexofendine LogP
Fexofendine Dosage Forms
Fexofendine Indication
Fexofendine Pharmacology
Fexofendine Absorption
Fexofendine side effects and Toxicity
Fexofendine Patient Information
Patients taking ALLEGRA tablets should receive the following information:
ALLEGRA tablets are prescribed for the relief of symptoms of seasonal allergic rhinitis or for the relief of symptoms of chronic idiopathic urticaria (hives). Patients should be instructed to take ALLEGRA only as prescribed. Do not exceed the recommended dose. If any untoward effects occur while taking ALLEGRA, discontinue use and consult the doctor.
The product should not be used by patients who are hypersensitive to it or to any of its ingredients.
Patients should be told that this product should be used in pregnancy or lactation only if the potential benefit justifies the potential risk to the fetus or nursing infant.
Patients should be advised to take the tablet with water. Patients should also be advised to store the medication in a tightly closed container in a cool, dry place, away from children.