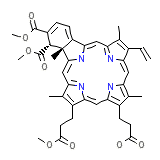
F3DThd




F3DThd Brand names, F3DThd Analogs
F3DThd Brand Names Mixture
- No information avaliable
F3DThd Chemical_Formula
C41H42N4O8
F3DThd RX_link
http://www.rxlist.com/cgi/generic2/verteporfin.htm
F3DThd fda sheet
F3DThd msds (material safety sheet)
F3DThd Synthesis Reference
No information avaliable
F3DThd Molecular Weight
718.794 g/mol
F3DThd Melting Point
No information avaliable
F3DThd H2O Solubility
No information avaliable
F3DThd State
Solid
F3DThd LogP
3.743
F3DThd Dosage Forms
Powder for solution to be mixed for injection (2mg verteporfin/mL reconstituted verteporfin)
F3DThd Indication
For the treatment of patients with predominantly classic subfoveal choroidal neovascularization due to age-related macular degeneration, pathologic myopia or presumed ocular histoplasmosis.
F3DThd Pharmacology
Verteporfin, otherwise known as benzoporphyrin derivative, is a medication used in conjunction with laser treatment to eliminate the abnormal blood vessels in the eye associated with conditions such as the wet form of macular degeneration. Verteporfin accumulates in these abnormal blood vessels and, when stimulated by nonthermal red light with a wavelength of 693 nm in the presence of oxygen, produces highly reactive short-lived singlet oxygen and other reactive oxygen radicals, resulting in local damage to the endothelium and blockage of the vessels.
F3DThd Absorption
No information avaliable
F3DThd side effects and Toxicity
Overdose of drug and/or light in the treated eye may result in nonperfusion of normal retinal vessels with the possibility of severe decrease in vision that could be permanent. An overdose of drug will also result in the prolongation of the period during which the patient remains photosensitive to bright light.
F3DThd Patient Information
F3DThd Organisms Affected
Humans and other mammals