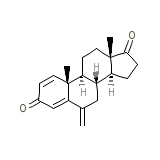
Exemestane




Exemestane Brand names, Exemestane Analogs
Exemestane Brand Names Mixture
- No information avaliable
Exemestane Chemical_Formula
C20H24O2
Exemestane RX_link
http://www.rxlist.com/cgi/generic3/exemest.htm
Exemestane fda sheet
Exemestane msds (material safety sheet)
Exemestane Synthesis Reference
Pfizer Canada Inc, Patent Number: 1277656
Exemestane Molecular Weight
296.403 g/mol
Exemestane Melting Point
155.13oC
Exemestane H2O Solubility
Non-soluble
Exemestane State
Solid
Exemestane LogP
4.222
Exemestane Dosage Forms
Tablet
Exemestane Indication
For the treatment of advanced breast cancer in postmenopausal women whose disease has progressed following tamoxifen therapy.
Exemestane Pharmacology
Aromatase is an enzyme that converts hormones to estrogen in the body's adrenal glands. The aromatase inhibitors (AIs) are drugs that reduce estrogen levels by blocking the action of aromatase in the adrenal glands. The selective AIs (SAIs) selectively reduce levels of estrogen without interfering with levels of other steroid hormones that are produced by the adrenal gland. Drugs in this class include anastrozole (Arimidex ™), letrozole (Femara ™) and exemestane (Aromasin ™).
Exemestane Absorption
42%
Exemestane side effects and Toxicity
Convulsions
Exemestane Patient Information
PATIENT INFORMATION
If clinically advisable, patients receiving OxyContin� (oxycodone hydrochloride controlled-release) tablets or
their caregivers should be given the following information by the physician, nurse, pharmacist, or caregiver:
1. Patients should be aware that OxyContin� tablets contain oxycodone, which is a morphine-like substance.
2. Patients should be advised that OxyContin� tablets were designed to work properly only if swallowed whole.
OxyContin� tablets will release all their contents at once if broken, chewed, or crushed, resulting in a risk of
fatal overdose.
3. Patients should be advised to report episodes of breakthrough pain and adverse experiences occurring during
therapy. Individualization of dosage is essential to make optimal use of this medication.
4. Patients should be advised not to adjust the dose of OxyContin� without consulting the prescribing professional.
5. Patients should be advised that OxyContin� may impair mental and/or physical ability required for the performance
of potentially hazardous tasks (e.g., driving, operating heavy machinery).
6. Patients should not combine OxyContin� with alcohol or other central nervous system depressants (sleep aids,
tranquilizers) except by the orders of the prescribing physician, because dangerous additive effects may occur,
resulting in serious injury or death.
7. Women of childbearing potential who become, or are planning to become, pregnant should be advised to consult their
physician regarding the effects of analgesics and other drug use during pregnancy on themselves and their unborn child.
8. Patients should be advised that OxyContin� is a potential drug of abuse. They should protect it from theft, and it
should never be given to anyone other than the individual for whom it was prescribed.
9. Patients should be advised that they may pass empty matrix "ghosts" (tablets) via colostomy or in the stool, and that
this is of no concern since the active medication has already been absorbed.
10. Patients should be advised that if they have been receiving treatment with OxyContin� for more than a few weeks and
cessation of therapy is indicated, it may be appropriate to taper the OxyContin� dose, rather than abruptly discontinue
it, due to the risk of precipitating withdrawal symptoms. Their physician can provide a dose schedule to accomplish a
gradual discontinuation of the medication.
11. Patients should be instructed to keep OxyContin� in a secure place out of the reach of children. When OxyContin� is
no longer needed, the unused tablets should be destroyed by flushing down the toilet.
If clinically advisable, patients receiving OxyContin� (oxycodone hydrochloride controlled-release) tablets or
their caregivers should be given the following information by the physician, nurse, pharmacist, or caregiver:
1. Patients should be aware that OxyContin� tablets contain oxycodone, which is a morphine-like substance.
2. Patients should be advised that OxyContin� tablets were designed to work properly only if swallowed whole.
OxyContin� tablets will release all their contents at once if broken, chewed, or crushed, resulting in a risk of
fatal overdose.
3. Patients should be advised to report episodes of breakthrough pain and adverse experiences occurring during
therapy. Individualization of dosage is essential to make optimal use of this medication.
4. Patients should be advised not to adjust the dose of OxyContin� without consulting the prescribing professional.
5. Patients should be advised that OxyContin� may impair mental and/or physical ability required for the performance
of potentially hazardous tasks (e.g., driving, operating heavy machinery).
6. Patients should not combine OxyContin� with alcohol or other central nervous system depressants (sleep aids,
tranquilizers) except by the orders of the prescribing physician, because dangerous additive effects may occur,
resulting in serious injury or death.
7. Women of childbearing potential who become, or are planning to become, pregnant should be advised to consult their
physician regarding the effects of analgesics and other drug use during pregnancy on themselves and their unborn child.
8. Patients should be advised that OxyContin� is a potential drug of abuse. They should protect it from theft, and it
should never be given to anyone other than the individual for whom it was prescribed.
9. Patients should be advised that they may pass empty matrix "ghosts" (tablets) via colostomy or in the stool, and that
this is of no concern since the active medication has already been absorbed.
10. Patients should be advised that if they have been receiving treatment with OxyContin� for more than a few weeks and
cessation of therapy is indicated, it may be appropriate to taper the OxyContin� dose, rather than abruptly discontinue
it, due to the risk of precipitating withdrawal symptoms. Their physician can provide a dose schedule to accomplish a
gradual discontinuation of the medication.
11. Patients should be instructed to keep OxyContin� in a secure place out of the reach of children. When OxyContin� is
no longer needed, the unused tablets should be destroyed by flushing down the toilet.
Exemestane Organisms Affected
Humans and other mammals