Etrafon-Forte




Etrafon-Forte Brand names, Etrafon-Forte Analogs
Etrafon-Forte Brand Names Mixture
- Apo Peram Tab 2-25 (Amitriptyline Hydrochloride + Perphenazine)
- Apo Peram Tab 3-15 (Amitriptyline Hydrochloride + Perphenazine)
- Elavil Plus Tab (Amitriptyline Hydrochloride + Perphenazine)
- Etrafon 2 10 (Amitriptyline Hydrochloride + Perphenazine)
- Etrafon a Tab (Amitriptyline Hydrochloride + Perphenazine)
- Etrafon D Tab (Amitriptyline Hydrochloride + Perphenazine)
- Etrafon F Tab (Amitriptyline Hydrochloride + Perphenazine)
- Pms-Levazine 2/25 Tab (Amitriptyline Hydrochloride + Perphenazine)
- Pms-Levazine 3/15 Tab (Amitriptyline Hydrochloride + Perphenazine)
- Pms-Levazine 4/25 Tab (Amitriptyline Hydrochloride + Perphenazine)
- Proavil Tab (Amitriptyline Hydrochloride + Perphenazine)
- Triavil Tab (Amitriptyline Hydrochloride + Perphenazine)
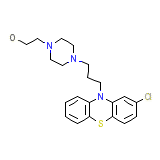
Etrafon-Forte Chemical_Formula
C21H26ClN3OS
Etrafon-Forte RX_link
http://www.rxlist.com/cgi/generic3/perphenazine.htm
Etrafon-Forte fda sheet
Etrafon-Forte msds (material safety sheet)
Etrafon-Forte Synthesis Reference
Cusic, U.S. pat. 2860138
Etrafon-Forte Molecular Weight
403.969 g/mol
Etrafon-Forte Melting Point
97 oC
Etrafon-Forte H2O Solubility
28.3 mg/L
Etrafon-Forte State
Solid
Etrafon-Forte LogP
4.176
Etrafon-Forte Dosage Forms
Liquid; Solution; Syrup; Tablet (2, 4, 8, or 16 mg)
Etrafon-Forte Indication
For use in the management of the manifestations of psychotic disorders and for the control of severe nausea and vomiting in adults.
Etrafon-Forte Pharmacology
Perphenazine is a piperazinyl phenothiazine, acts on the central nervous system, and has a greater behavioral potency than other phenothiazine derivatives whose side chains do not contain a piperazine moiety. It is a member of a class of drugs called phenothiazines, which are dopamine D1/D2 receptor antagonists. Perphenazine is 10 to 15 times as potent as chlorpromazine; that means perphenazine is a highly potent antipsychotic. In equivalent doses it has approximately the same frequency and severity of early and late extrapypramidal side-effects compared to Haloperidol.
Etrafon-Forte Absorption
Absolute bioavailability is 40% following oral administration.
Etrafon-Forte side effects and Toxicity
Symptoms of overdose include stupor or coma, and children may have convulsive seizures. Signs of arousal may not occur for 48 hours. Oral LD50=318 mg/kg (rat); IPR LD50=64 mg/kg (mouse)
Etrafon-Forte Patient Information
This information is intended to aid in the safe and effective use of this medication. It is not a disclosure of all possible adverse or intended effects.
Given the likelihood that a substantial proportion of patients exposed chronically to neuroleptics will develop tardive dyskinesia, it is advised that all patients in whom chronic use is contemplated be given, if possible, full information about this risk. The decision to inform patients and/or their guardians must obviously take into account the clinical circumstances and the competency of the patient to understand the information provided.
Etrafon-Forte Organisms Affected
Humans and other mammals