Endovalpin




Endovalpin Brand names, Endovalpin Analogs
Endovalpin Brand Names Mixture
- No information avaliable
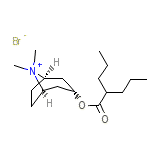
Endovalpin Chemical_Formula
C17H32BrNO2
Endovalpin RX_link
No information avaliable
Endovalpin fda sheet
Endovalpin msds (material safety sheet)
Endovalpin Synthesis Reference
No information avaliable
Endovalpin Molecular Weight
362.346 g/mol
Endovalpin Melting Point
329 oC
Endovalpin H2O Solubility
1600 mg/L
Endovalpin State
Solid
Endovalpin LogP
0.60
Endovalpin Dosage Forms
Tablet (oral, 50mg)
Endovalpin Indication
For use in conjunction with antacids or histamine H2-receptor antagonists in the treatment of peptic ulcer, to reduce further gastric acid secretion and delay gastric emptying.
Endovalpin Pharmacology
Anisotropine methylbromide is a quaternary ammonium compound. Its use as treatment adjunct in peptic ulcer has been replaced by the use of more effective agents. Depending on the dose, anisotropine methylbromide may reduce the motility and secretory activity of the gastrointestinal system, and the tone of the ureter and urinary bladder and may have a slight relaxant action on the bile ducts and gallbladder. In general, smaller doses of anisotropine methylbromide inhibit salivary and bronchial secretions, sweating, and accommodation; cause dilatation of the pupil; and increase the heart rate. Larger doses are required to decrease motility of the gastrointestinal and urinary tracts and to inhibit gastric acid secretion.
Endovalpin Absorption
Gastrointestinal absorption is poor and irregular. Total absorption after an oral dose is about 10 to 25%.
Endovalpin side effects and Toxicity
No information avaliable
Endovalpin Patient Information
Endovalpin Organisms Affected
Humans and other mammals