Ecostatin Vaginal Ovules




Ecostatin Vaginal Ovules Brand names, Ecostatin Vaginal Ovules Analogs
Ecostatin Vaginal Ovules Brand Names Mixture
- No information avaliable
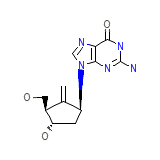
Ecostatin Vaginal Ovules Chemical_Formula
C12H15N5O3
Ecostatin Vaginal Ovules RX_link
http://www.rxlist.com/cgi/generic4/baraclude.htm
Ecostatin Vaginal Ovules fda sheet
Ecostatin Vaginal Ovules msds (material safety sheet)
Ecostatin Vaginal Ovules Synthesis Reference
No information avaliable
Ecostatin Vaginal Ovules Molecular Weight
277.279 g/mol
Ecostatin Vaginal Ovules Melting Point
No information avaliable
Ecostatin Vaginal Ovules H2O Solubility
Slightly soluble (2.4 mg/mL at pH 7.9, 25oC)
Ecostatin Vaginal Ovules State
Solid
Ecostatin Vaginal Ovules LogP
No information avaliable
Ecostatin Vaginal Ovules Dosage Forms
Film-coated tablets for oral administration (0.5 mg, 1 mg); Oral solution (0.05 mg/mL)
Ecostatin Vaginal Ovules Indication
For the treatment of chronic hepatitis B virus infection in adults with evidence of active viral replication and either evidence of persistent elevations in serum aminotransferases (ALT or AST) or histologically active disease.
Ecostatin Vaginal Ovules Pharmacology
Entecavir is a guanosine nucleoside analogue with selective activity against hepatitis B virus (HBV). It is designed to selectively inhibit the Hepatitis B virus, blocking all three steps in the replication process. Entecavir is more efficient than an older Hepatitis B drug, lamivudine.
Ecostatin Vaginal Ovules Absorption
Absorption Following oral administration in healthy subjects, entecavir peak plasma concentrations occurred between 0.5 and 1.5 hours. In healthy subjects, the bioavailability of the tablet is 100% relative to the oral solution.
Ecostatin Vaginal Ovules side effects and Toxicity
No information avaliable
Ecostatin Vaginal Ovules Patient Information
Ecostatin Vaginal Ovules Organisms Affected
Hepatitis B