Ebixa




Ebixa Brand names, Ebixa Analogs
Ebixa Brand Names Mixture
- No information avaliable
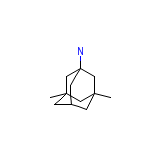
Ebixa Chemical_Formula
C12H21N
Ebixa RX_link
http://www.rxlist.com/cgi/generic3/namenda.htm
Ebixa fda sheet
Ebixa msds (material safety sheet)
Ebixa Synthesis Reference
No information avaliable
Ebixa Molecular Weight
179.302 g/mol
Ebixa Melting Point
258 oC (HCl salt)
Ebixa H2O Solubility
35 mg/mL (HCl salt), 0.9 mg/mL for free base
Ebixa State
Solid
Ebixa LogP
2.197
Ebixa Dosage Forms
Tablet
Ebixa Indication
For the treatment of moderate to severe dementia of the Alzheimer's type.
Ebixa Pharmacology
Memantine, an amantadine derivative, is an NMDA receptor antagonist used in the treatment of Alzheimer's disease. It differs from traditional agents used in Alzheimer's disease by acting on glutamatergic neurotransmission, rather than cholinergic. There is some evidence that dysfunction of glutamatergic neurotransmission, manifested as neuronal excitotoxicity, is involved in the aetiology of Alzheimer's disease (Cacabelos et al., 1999). As such, targeting the glutamatergic system, specifically NMDA receptors, was a novel approach to treatment in view of the limited efficacy of existing drugs targeting the cholinergic system. A systematic review of randomised controlled trials found that memantine has a positive effect on cognition, mood, behaviour, and the ability to perform daily activities. There is no evidence that memantine prevents or slows neurodegeneration in patients with Alzheimer's disease.
Ebixa Absorption
Well absorbed orally with a bioavailability of approximately 100%. Peak plasma concentrations are reached in 3-7 hours. Food has no effect on absorption.
Ebixa side effects and Toxicity
Side effects include pain, abnormal crying, leg pain, fever, increased apetite. Adverse drug reactions include: dizziness, confusion, headache, hallucinations, tiredness. Less common side effects include: vomiting, anxiety, hypertonia, cystitis, and increased libido. Doses of up to 400 mg have been tolerated.
Ebixa Patient Information
Information for Patients and Caregivers: Caregivers should be instructed in the recommended administration (twice per day for doses above 5 mg) and dose escalation (minimum interval of one week between dose increases).
Ebixa Organisms Affected
Humans and other mammals