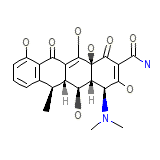
Doxychel Hyclate




Doxychel Hyclate Brand names, Doxychel Hyclate Analogs
Doxychel Hyclate Brand Names Mixture
- No information avaliable
Doxychel Hyclate Chemical_Formula
C22H24N2O8
Doxychel Hyclate RX_link
http://www.rxlist.com/cgi/generic/doxycyc.htm
Doxychel Hyclate fda sheet
Doxychel Hyclate msds (material safety sheet)
Doxychel Hyclate Synthesis Reference
C. R. Stephens et al.; J. Am. Chem. Soc. 80; 5324 (1958)
Doxychel Hyclate Molecular Weight
444.435 g/mol
Doxychel Hyclate Melting Point
201 oC
Doxychel Hyclate H2O Solubility
630 mg/L
Doxychel Hyclate State
Solid
Doxychel Hyclate LogP
-1.219
Doxychel Hyclate Dosage Forms
Tablet; Capsule; Gel; Liquid; Powder; Powder for solution
Doxychel Hyclate Indication
For the treatment of the following infections:Rocky mountain spotted fever, typhus fever and the typhus group, Q fever, rickettsialpox, and tick fevers caused by Rickettsiae, Chancroid caused by Haemophilus ducreyi, Uncomplicated gonorrhea caused by Neisseria gonorrhoeae, Respiratory tract infections caused by Mycoplasma pneumoniae or Streptococcus pneumoniae. Lymphogranuloma ve
Doxychel Hyclate Pharmacology
Doxycycline, a long-acting tetracycline derived from oxytetracycline, is used to inhibit bacterial protein synthesis and treat non-gonococcal urethritis and cervicitis, exacerbations of bronchitis in patients with COPD, and adult periodontitis.
Doxychel Hyclate Absorption
completely absorbed after oral administration
Doxychel Hyclate side effects and Toxicity
anorexia, nausea, diarrhoea, glossitis, dysphagia, enterocolitis and inflammatory lesions (with monilial overgrowth) in the anogenital region. skin reactions such as maculopapular and erythematous rashes, exfoliative dermatitis, photosensitivity, hypersensitivity reactions such as urticaria, angioneurotic oedema, anaphylaxis, anaphyl-actoid purpura, pericarditis, and exacerbation of systemic lupus erythematosus, benign intracranial hypertension in adults disappearing on discontinuation of the medicine, haematologic abnormalities such as haemolytic anaemia, thrombocytopenia, neutropenia, and eosinophilia. LD50=262 mg/kg(i.p. in rat)
Doxychel Hyclate Patient Information
Doxychel Hyclate Organisms Affected
Enteric bacteria and other eubacteria