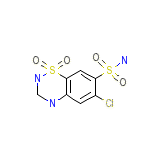
Dihydrochlorothiazidum




Dihydrochlorothiazidum Brand names, Dihydrochlorothiazidum Analogs
- Acuretic
- Aldactazide
- Aldoril
- Apresazide
- Aquarills
- Aquarius
- Bremil
- Caplaril
- Capozide
- Chlorosulthiadil
- Chlorothiazide
- Chlorsulfonamidodihydrobenzothiadiazine Dioxide
- Chlorzide
- Cidrex
- Dichlorosal
- Dichlorotride
- Dichlotiazid
- Dichlotride
- Diclotride
- Dicyclotride
- Dihydrochlorothiazid
- Dihydrochlorothiazide
- Dihydrochlorothiazidum
- Dihydrochlorurit
- Dihydrochlorurite
- Dihydroxychlorothiazidum
- Direma
- Disalunil
- Diu-Melusin
- Diuril
- Drenol
- Dyazide
- Esidrex
- Esidrix
- Esimil
- Fluvin
- HCTZ
- HCZ
- Hidril
- Hidrochlortiazid
- Hidroronol
- Hidrotiazida
- Hydril
- Hydro-Aquil
- Hydro-D
- Hydro-Diuril
- Hydrochlorothiazid
- Hydrochlorothiazide Intensol
- Hydrochlorthiazide
- Hydrodiuretic
- Hydrodiuril
- Hydropres
- Hydrosaluric
- Hydrothide
- Hydrozide
- Hypothiazid
- Hypothiazide
- Hyzaar
- Idrotiazide
- Inderide
- Ivaugan
- Jen-Diril
- Lopressor Hct
- Lotensin Hct
- Maschitt
- Maxzide
- Megadiuril
- Microzide
- Moduretic
- Nefrix
- Neo-Codema
- Neoflumen
- Newtolide
- Oretic
- Palonyl
- Panurin
- Perovex
- Primogyn
- Prinzide
- Ro-Hydrazide
- Servithiazid
- Thiaretic
- Thiuretic
- Thlaretic
- Timolide
- Unipres
- Urodiazin
- Vaseretic
- Vetidrex
- Ziac
- Zide
Dihydrochlorothiazidum Brand Names Mixture
- No information avaliable
Dihydrochlorothiazidum Chemical_Formula
C7H8ClN3O4S2
Dihydrochlorothiazidum RX_link
http://www.rxlist.com/cgi/generic/hctz.htm
Dihydrochlorothiazidum fda sheet
Dihydrochlorothiazidum msds (material safety sheet)
Dihydrochlorothiazidum Synthesis Reference
Werner et al., J. Am. Chem. Soc. 82, 1161 (1960)
Dihydrochlorothiazidum Molecular Weight
297.741 g/mol
Dihydrochlorothiazidum Melting Point
274 oC
Dihydrochlorothiazidum H2O Solubility
0.7 mg/mL
Dihydrochlorothiazidum State
Solid
Dihydrochlorothiazidum LogP
-0.268
Dihydrochlorothiazidum Dosage Forms
Tablet (oral)
Dihydrochlorothiazidum Indication
For the treatment of high blood pressure and management of edema.
Dihydrochlorothiazidum Pharmacology
Thiazides such as hydrochlorothiazide promote water loss from the body (diuretics). They inhibit Na+/Cl- reabsorption from the distal convoluted tubules in the kidneys. Thiazides also cause loss of potassium and an increase in serum uric acid. Thiazides are often used to treat hypertension, but their hypotensive effects are not necessarily due to their diuretic activity. Thiazides have been shown to prevent hypertension-related morbidity and mortality although the mechanism is not fully understood. Thiazides cause vasodilation by activating calcium-activated potassium channels (large conductance) in vascular smooth muscles and inhibiting various carbonic anhydrases in vascular tissue.
Dihydrochlorothiazidum Absorption
50-60%
Dihydrochlorothiazidum side effects and Toxicity
The most common signs and symptoms observed are those caused by electrolyte depletion (hypokalemia, hypochloremia, hyponatremia) and dehydration resulting from excessive diuresis. If digitalis has also been administered, hypokalemia may accentuate cardiac arrhythmias. The oral LD50 of hydrochlorothiazide is greater than 10 g/kg in the mouse and rat.
Dihydrochlorothiazidum Patient Information
General
All patients receiving diuretic therapy should be observed for evidence of fluid or electrolyte
imbalance: namely, hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine
electrolyte determinations are particularly important when the patient is vomiting excessively or
receiving parenteral fluids. Warning signs or symptoms of fluid and electrolyte imbalance,
irrespective of cause, include dryness of mouth, thirst, weakness, lethargy, drowsiness,
restlessness, confusion, seizures, muscle pains or cramps, muscular fatigue, hypotension,
oliguria, tachycardia, and gastrointestinal disturbance such as nausea or vomiting.
Hypokalemia may develop, especially with brisk diuresis, when severe cirrhosis is present or
after prolonged therapy.
Interference with adequate oral electrolyte intake will also contribute to hypokalemia.
Hypokalemia may cause cardiac arrhythmia and may also sensitize or exaggerate the response of
the heart to the toxic effects of digitalis (e.g., increased ventricular irritability).
Hypokalemia may be avoided or treated by use of potassium sparing diuretics or potassium
supplements such as foods with a high potassium content.
Although any chloride deficit is generally mild and usually does not require specific treatment
except under extraordinary circumstances (as in liver disease or renal disease), chloride
replacement may be required in the treatment of metabolic alkalosis.
Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is
water restriction, rather than administration of salt, except in rare instances when the
hyponatremia is life threatening. In actual salt depletion, appropriate replacement is the
therapy of choice.
Hyperuricemia may occur or acute gout may be precipitated in certain patients receiving thiazides.
In diabetic patients dosage adjustments of insulin or oral hypoglycemic agents may be required.
Hyperglycemia may occur with thiazide diuretics. Thus latent diabetes mellitus may become manifest
during thiazide therapy.
The antihypertensive effects of the drug may be enhanced in the post-sympathectomy patient.
If progressive renal impairment becomes evident, consider withholding or discontinuing diuretic therapy.
Thiazides have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
Thiazides may decrease urinary calcium excretion. Thiazides may cause intermittent and slight elevation
of serum calcium in the absence of known disorders of calcium metabolism. Marked hypercalcemia may be
evidence of hidden hyperparathyroidism. Thiazides should be discontinued before carrying out tests for
parathyroid function.
Increases in cholesterol and triglyceride levels may be associated with thiazide diuretic therapy.
Laboratory Tests
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be done
at appropriate intervals.
All patients receiving diuretic therapy should be observed for evidence of fluid or electrolyte
imbalance: namely, hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine
electrolyte determinations are particularly important when the patient is vomiting excessively or
receiving parenteral fluids. Warning signs or symptoms of fluid and electrolyte imbalance,
irrespective of cause, include dryness of mouth, thirst, weakness, lethargy, drowsiness,
restlessness, confusion, seizures, muscle pains or cramps, muscular fatigue, hypotension,
oliguria, tachycardia, and gastrointestinal disturbance such as nausea or vomiting.
Hypokalemia may develop, especially with brisk diuresis, when severe cirrhosis is present or
after prolonged therapy.
Interference with adequate oral electrolyte intake will also contribute to hypokalemia.
Hypokalemia may cause cardiac arrhythmia and may also sensitize or exaggerate the response of
the heart to the toxic effects of digitalis (e.g., increased ventricular irritability).
Hypokalemia may be avoided or treated by use of potassium sparing diuretics or potassium
supplements such as foods with a high potassium content.
Although any chloride deficit is generally mild and usually does not require specific treatment
except under extraordinary circumstances (as in liver disease or renal disease), chloride
replacement may be required in the treatment of metabolic alkalosis.
Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is
water restriction, rather than administration of salt, except in rare instances when the
hyponatremia is life threatening. In actual salt depletion, appropriate replacement is the
therapy of choice.
Hyperuricemia may occur or acute gout may be precipitated in certain patients receiving thiazides.
In diabetic patients dosage adjustments of insulin or oral hypoglycemic agents may be required.
Hyperglycemia may occur with thiazide diuretics. Thus latent diabetes mellitus may become manifest
during thiazide therapy.
The antihypertensive effects of the drug may be enhanced in the post-sympathectomy patient.
If progressive renal impairment becomes evident, consider withholding or discontinuing diuretic therapy.
Thiazides have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
Thiazides may decrease urinary calcium excretion. Thiazides may cause intermittent and slight elevation
of serum calcium in the absence of known disorders of calcium metabolism. Marked hypercalcemia may be
evidence of hidden hyperparathyroidism. Thiazides should be discontinued before carrying out tests for
parathyroid function.
Increases in cholesterol and triglyceride levels may be associated with thiazide diuretic therapy.
Laboratory Tests
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be done
at appropriate intervals.
Dihydrochlorothiazidum Organisms Affected
Humans and other mammals