Detrol LA




Detrol LA Brand names, Detrol LA Analogs
Detrol LA Brand Names Mixture
- No information avaliable
Detrol LA Chemical_Formula
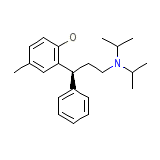
C22H31NO
Detrol LA RX_link
http://www.rxlist.com/cgi/generic2/tolter.htm
Detrol LA fda sheet
Detrol LA msds (material safety sheet)
Detrol LA Synthesis Reference
No information avaliable
Detrol LA Molecular Weight
325.488 g/mol
Detrol LA Melting Point
No information avaliable
Detrol LA H2O Solubility
No information avaliable
Detrol LA State
Solid
Detrol LA LogP
6.076
Detrol LA Dosage Forms
Capsule (sustained-release)
Detrol LA Indication
For the treatment of overactive bladder (with symptoms of urinary frequency, urgency, or urge incontinence).
Detrol LA Pharmacology
Tolterodine is a competitive muscarinic receptor antagonist. Both urinary bladder contraction and salivation are mediated via cholinergic muscarinic receptors. After oral administration, tolterodine is metabolized in the liver, resulting in the formation of the 5-hydroxymethyl derivative, a major pharmacologically active metabolite. The 5-hydroxymethyl metabolite, which exhibits an antimuscarinic activity similar to that of tolterodine, contributes significantly to the therapeutic effect. Both tolterodine and the 5-hydroxymethyl metabolite exhibit a high specificity for muscarinic receptors, since both show negligible activity or affinity for other neurotransmitter receptors and other potential cellular targets, such as calcium channels. Tolterodine has a pronounced effect on bladder function. The main effects of tolterodine are an increase in residual urine, reflecting an incomplete emptying of the bladder, and a decrease in detrusor pressure, consistent with an antimuscarinic action on the lower urinary tract.
Detrol LA Absorption
No information avaliable
Detrol LA side effects and Toxicity
No information avaliable
Detrol LA Patient Information
No information avaliable
Detrol LA Organisms Affected
Humans and other mammals