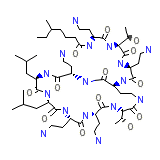
Demykon




Demykon Brand names, Demykon Analogs
Demykon Brand Names Mixture
- No information avaliable
Demykon Chemical_Formula
C52H98N16O13
Demykon RX_link
No information avaliable
Demykon fda sheet
Demykon msds (material safety sheet)
Demykon Synthesis Reference
No information avaliable
Demykon Molecular Weight
2797.3193 g/mol
Demykon Melting Point
200 - 220 oC
Demykon H2O Solubility
5.64E+005 mg/L
Demykon State
Solid
Demykon LogP
-4.648
Demykon Dosage Forms
Capsule; Cream; Liquid; Ointment; Powder for solution; Solution; Suspension; Tablet; Tablet (enteric-coated); Tablet (extended-release)
Demykon Indication
For the treatment of acute or chronic infections due to sensitive strains of certain gram-negative bacilli, particularly Pseudomonas aeruginosa.
Demykon Pharmacology
Colistin is a polymyxin antibiotic agent. Polymyxins are cationic polypeptides that disrupt the bacterial cell membrane through a detergentlike mechanism. With the development of less toxic agents, such as extended-spectrum penicillins and cephalosporins, parenteral polymyxin use was largely abandoned, except for the treatment of multidrug-resistant pulmonary infections in patients with cystic fibrosis. More recently, however, the emergence of multidrug-resistant gram-negative bacteria, such as Pseudomonas aeruginosa and Acinetobacter baumannii, and the lack of new antimicrobial agents have led to the revived use of the polymyxins.
Demykon Absorption
Very poor absorption from gastrointestinal tract.
Demykon side effects and Toxicity
Oral LD50 in rats is 5450 mg/kg. Overdosage with colistimethate can cause neuromuscular blockade characterized by paresthesia, lethargy, confusion, dizziness, ataxia, nystagmus, disorders of speech and apnea. Respiratory muscle paralysis may lead to apnea, respiratory arrest and death.
Demykon Patient Information
No information avaliable
Demykon Organisms Affected
Gram-negative bacilli