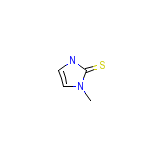
Danantizol




Danantizol Brand names, Danantizol Analogs
- Basolan
- Danantizol
- Favistan
- Frentirox
- Mercaptazole
- Mercasolyl
- Mercazole
- Mercazolyl
- Merkastan
- Merkazolil
- Metazolo
- Methamazole
- Methiamazole
- Methimazol
- Methylmercaptoimidazole
- Metizol
- Metothyrin
- Metothyrine
- Metotirin
- Propyl-Thyracil
- Strumazol
- Strumazole
- Tapazole
- Tapuzole
- Thacapzol
- Thiamazol
- Thiamazole
- Thimazole
- Thycapsol
- Thycapzol
- Thymidazol
- Thymidazole
- USAF EL-30
Danantizol Brand Names Mixture
- No information avaliable
Danantizol Chemical_Formula
C4H6N2S
Danantizol RX_link
http://www.rxlist.com/cgi/generic3/methim.htm
Danantizol fda sheet
Danantizol msds (material safety sheet)
Danantizol Synthesis Reference
No information avaliable
Danantizol Molecular Weight
114.17 g/mol
Danantizol Melting Point
146 oC
Danantizol H2O Solubility
275 g/L
Danantizol State
Solid
Danantizol LogP
-0.34
Danantizol Dosage Forms
Tablet (5 or 10 mg)
Danantizol Indication
For the treatment of hyperthyroidism, goiter, Graves disease and psoriasis.
Danantizol Pharmacology
Used in the treatment of hyperthyroidism or an overactive thyroid gland, methimazole inhibits the synthesis of thyroid hormones and thus is effective in the treatment of hyperthyroidism. It may also be used to ameliorate hyperthyroidism in preparation for subtotal thyroidectomy or radioactive iodine therapy.
Danantizol Absorption
Rapid with an oral bioavailability of 93%.
Danantizol side effects and Toxicity
Oral LD50 in rats is 2250 mg/kg. Symptoms of overdose include nausea, vomiting, epigastric distress, headache, fever, joint pain, pruritus, and edema. Aplastic anemia (pancy-topenia) or agranulocytosis may be manifested in hours to days. Less frequent events are hepatitis, nephrotic syndrome, exfoliative dermatitis, neuropathies, and CNS stimulation or depression.
Danantizol Patient Information
Patients should be instructed to report to their physicians any symptoms of agranulocytosis, such as fever or sore throat.
Danantizol Organisms Affected
Humans and other mammals