DEA No. 1610




DEA No. 1610 Brand names, DEA No. 1610 Analogs
- Adiposon
- Amfepramon
- Amfepramone
- Amfepramone HCL
- Amfepramone Hydrochloride
- Amfepramonum [INN-Latin]
- Amphepramon
- Amphepramone
- Amphepramonum hydrochloride
- Anfamon
- Anfepramona [INN-Spanish]
- Anorex
- Cegramine
- DEA No. 1610
- Danylen
- Derfon
- Diethylpropion HCL
- Diethylpropion Hydrochloride
- Diethylpropione
- Diethylpropione hydrochloride
- Dobesin
- Frekentine
- Keramik
- Keramin
- Magrene
- Moderatan
- Modulor
- Neobes
- Nopropiophenone
- Obesitex
- Parabolin
- Prefamone
- Regenon
- Regenon hydrochloride
- Reginon
- Silutin
- Tenuate
- Tenuate Dospan
- Tenuate hydrochloride
- Tepanil
- Tepanil Ten-tab
- Tylinal
- alpha-Benzoyltriethylamine
- alpha-Diethylaminopropiophenone
DEA No. 1610 Brand Names Mixture
- No information avaliable
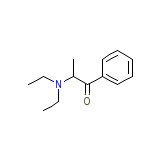
DEA No. 1610 Chemical_Formula
C13H19NO
DEA No. 1610 RX_link
http://www.rxlist.com/cgi/generic3/diethprop.htm
DEA No. 1610 fda sheet
DEA No. 1610 msds (material safety sheet)
DEA No. 1610 Synthesis Reference
No information avaliable
DEA No. 1610 Molecular Weight
205.296 g/mol
DEA No. 1610 Melting Point
No information avaliable
DEA No. 1610 H2O Solubility
No information avaliable
DEA No. 1610 State
No information avaliable
DEA No. 1610 LogP
2.951
DEA No. 1610 Dosage Forms
25 mg tablets and 75 mg controlled-release tablets.
DEA No. 1610 Indication
Used in the management of exogenous obesity as a short-term adjunct (a few weeks) in a regimen of weight reduction based on caloric restriction.
DEA No. 1610 Pharmacology
Diethylpropion is a sympathomimetic stimulant drug marketed as an appetite suppressant. Chemically, it is the N,N-diethyl analog of cathinone. Its mechanism of action is similar to other appetite suppressants such as sibutramine, phentermine and dextroamphetamine.
DEA No. 1610 Absorption
Diethylpropion is rapidly absorbed from the GI tract after oral administration.
DEA No. 1610 side effects and Toxicity
The reported oral LD50 for mice is 600 mg/kg, for rats is 250 mg/kg and for dogs is 225 mg/kg. Manifestation of acute overdosage include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, and panic states.
DEA No. 1610 Patient Information
No information avaliable
DEA No. 1610 Organisms Affected
Humans and other mammals