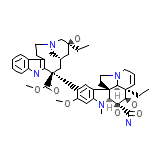
DAVA




DAVA Brand names, DAVA Analogs
DAVA Brand Names Mixture
- No information avaliable
DAVA Chemical_Formula
C43H55N5O7
DAVA RX_link
http://www.rxlist.com/cgi/generic2/vinor_cp.htm
DAVA fda sheet
DAVA msds (material safety sheet)
DAVA Synthesis Reference
G. J. Cullinan, K. Gerzon, Ger. pat. 2,415,980 (1974 to Lilly)
DAVA Molecular Weight
753.926 g/mol
DAVA Melting Point
230-232 oC
DAVA H2O Solubility
No information avaliable
DAVA State
Solid
DAVA LogP
4.002
DAVA Dosage Forms
IM solution; Powder for solution
DAVA Indication
For the treatment of acute leukaemia, malignant lymphoma, Hodgkin's disease, acute erythraemia and acute panmyelosis
DAVA Pharmacology
Vindesine is indicated for the treatment of acute lymphocytic leukemia of childhood that is resistant to vincristine and non-oat cell lung cancer.Vindesine causes the arrest of cells in metaphase mitosis. It is three times more potent than vincristine and nearly 10 times more potent than vinblastine in causing mitotic arrest in in vitro studies at doses designed to arrest from 10 to 15% of the cells in mitosis. Vindesine and vincristine are approximately equipotent at dose levels that arrest 40 to 50% of the cells in mitosis. Unlike vinblastine, vindesine produces very few postmetaphase cells. Vindesine has demonstrated activity in patients who have relapsed while receiving multiple-agent treatment that included vincristine.
DAVA Absorption
No information avaliable
DAVA side effects and Toxicity
No information avaliable
DAVA Patient Information
Patient Information:
The treatmewnt with Vindesine can cause hair, but it is usually reversible. It may also cause
loss of sensation or tingling in hands or feet, fever, chill, sore throat, constipation, black
stool, bruising or bleeding.
Patients should be informed that the major acute toxicities of Vindesine are related to bone
marrow toxicity, specifically granulocytopenia with increased susceptibility to infection.
They should be advised to report fever or chills immediately. Women of childbearing potential
should be advised to avoid becoming pregnant during treatment. Patients should be advised to
contact their physician if they experience increased shortness of breath, cough, or other new
pulmonary symptoms, or if they experience symptoms of abdominal pain or constipation.
The treatmewnt with Vindesine can cause hair, but it is usually reversible. It may also cause
loss of sensation or tingling in hands or feet, fever, chill, sore throat, constipation, black
stool, bruising or bleeding.
Patients should be informed that the major acute toxicities of Vindesine are related to bone
marrow toxicity, specifically granulocytopenia with increased susceptibility to infection.
They should be advised to report fever or chills immediately. Women of childbearing potential
should be advised to avoid becoming pregnant during treatment. Patients should be advised to
contact their physician if they experience increased shortness of breath, cough, or other new
pulmonary symptoms, or if they experience symptoms of abdominal pain or constipation.
DAVA Organisms Affected
Humans and other mammals