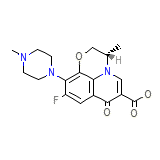
Cravit Ophthalmic




Cravit Ophthalmic Brand names, Cravit Ophthalmic Analogs
Cravit Ophthalmic Brand Names Mixture
- No information avaliable
Cravit Ophthalmic Chemical_Formula
C36H42F2N6O9
Cravit Ophthalmic RX_link
http://www.rxlist.com/cgi/generic2/quixin.htm
Cravit Ophthalmic fda sheet
Cravit Ophthalmic msds (material safety sheet)
Cravit Ophthalmic Synthesis Reference
Mitscher, Lester A. et al.; J.Med.Chem.; 30; 12; 2283-2286(1987)
Cravit Ophthalmic Molecular Weight
740.751 g/mol
Cravit Ophthalmic Melting Point
No information avaliable
Cravit Ophthalmic H2O Solubility
Insoluble
Cravit Ophthalmic State
Solid
Cravit Ophthalmic LogP
1.268
Cravit Ophthalmic Dosage Forms
Tablet; Liquid; Solution
Cravit Ophthalmic Indication
For the treatment of bacterial conjunctivitis caused by susceptible strains of the following organisms: Corynebacterium species, Staphylococus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, Streptococcus (Groups C/F/G), Viridans group streptococci, Acinetobacter lwoffii, Haemophilus influenzae, Serratia marcescens.
Cravit Ophthalmic Pharmacology
Levofloxacin, a fluoroquinolone antiinfective, is the optically active L-isomer of ofloxacin. Levofloxacin is used to treat bacterial conjunctivitis, sinusitis, chronic bronchitis, community-acquired pneumonia and pneumonia caused by penicillin-resistant strains of Streptococcus pneumoniae, skin and skin structure infections, complicated urinary tract infections and acute pyelonephritis.
Cravit Ophthalmic Absorption
Absorption of ofloxacin after single or multiple doses of 200 to 400 mg is predictable, and the amount of drug absorbed increases proportionately with the dose.
Cravit Ophthalmic side effects and Toxicity
Side effects include disorientation, dizziness, drowsiness, hot and cold flashes, nausea, slurring of speech, swelling and numbness in the face
Cravit Ophthalmic Patient Information
PATIENT INFORMATION
While taking ofloxacin patient should be advised to:
� to drink fluids liberally;
� that mineral supplements, vitamins with iron or minerals, calcium- , aluminum-, or magnesium-based antacids, sucralfate or Videx�, (Didanosine), chewable/buffered tablets or the pediatric powder for oral solution should not be taken within the two-hour period before or within the two-hour period after taking ofloxacin;
� that ofloxacin can be taken without regard to meals;
� that ofloxacin may cause neurologic adverse effects (e. g. , dizziness, lightheadedness) and that patients should know how they react to ofloxacin before they operate an automobile or machinery or engage in activities requiring mental alertness and coordination;
� to discontinue treatment and inform their physician if they experience pain, inflammation, or rupture of a tendon, and to rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture has been confidently excluded;
� that ofloxacin may be associated with hypersensitivity reactions, even following the first dose, to discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat, difficulty in swallowing or breathing, any swelling suggesting angioedema (e. g. , swelling of the lips, tongue, face; tightness of the throat, hoarseness), or any other symptom of an allergic reaction
� to avoid excessive sunlight or artificial ultraviolet light while receiving ofloxacin and to discontinue therapy if phototoxicity (e. g. , skin eruption) occurs;
� that if they are diabetic and are being treated with insulin or an oral hypoglycemic drug, to discontinue ofloxacin immediately if a hypoglycemic reaction occurs and consult a physician;
� that convulsions have been reported in patients taking quinolones, including ofloxacin, and to notify their physician before taking this drug if there is a history of this condition.
While taking ofloxacin patient should be advised to:
� to drink fluids liberally;
� that mineral supplements, vitamins with iron or minerals, calcium- , aluminum-, or magnesium-based antacids, sucralfate or Videx�, (Didanosine), chewable/buffered tablets or the pediatric powder for oral solution should not be taken within the two-hour period before or within the two-hour period after taking ofloxacin;
� that ofloxacin can be taken without regard to meals;
� that ofloxacin may cause neurologic adverse effects (e. g. , dizziness, lightheadedness) and that patients should know how they react to ofloxacin before they operate an automobile or machinery or engage in activities requiring mental alertness and coordination;
� to discontinue treatment and inform their physician if they experience pain, inflammation, or rupture of a tendon, and to rest and refrain from exercise until the diagnosis of tendinitis or tendon rupture has been confidently excluded;
� that ofloxacin may be associated with hypersensitivity reactions, even following the first dose, to discontinue the drug at the first sign of a skin rash, hives or other skin reactions, a rapid heartbeat, difficulty in swallowing or breathing, any swelling suggesting angioedema (e. g. , swelling of the lips, tongue, face; tightness of the throat, hoarseness), or any other symptom of an allergic reaction
� to avoid excessive sunlight or artificial ultraviolet light while receiving ofloxacin and to discontinue therapy if phototoxicity (e. g. , skin eruption) occurs;
� that if they are diabetic and are being treated with insulin or an oral hypoglycemic drug, to discontinue ofloxacin immediately if a hypoglycemic reaction occurs and consult a physician;
� that convulsions have been reported in patients taking quinolones, including ofloxacin, and to notify their physician before taking this drug if there is a history of this condition.
Cravit Ophthalmic Organisms Affected
Enteric bacteria and other eubacteria