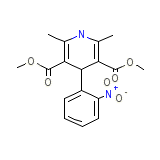
Corynphar




Corynphar Brand names, Corynphar Analogs
- Adalat
- Adalat 10
- Adalat 20
- Adalat 5
- Adalat CC
- Adalat CR
- Adalat Crono
- Adalat Ft
- Adalat Gits
- Adalat Gits 30
- Adalat LA
- Adalat LP
- Adalat Oros
- Adalat PA
- Adalat Retard
- Adalate
- Adapine
- Adapress
- Alat
- Aldipin
- Alfadal
- Alonix
- Alonix S
- Alpha-Nifedipine Retard
- Angipec
- Anifed
- Anpine
- Apo-Nifed
- Aprical
- Bonacid
- Calcibloc
- Calcigard
- Calcilat
- Camont
- Cardifen
- Cardilat
- Cardilate
- Cardionorm
- Chronadalate
- Chronadalate Lp
- Citilat
- Coracten
- Coral
- Cordafen
- Cordaflex
- Cordalat
- Cordicant
- Cordilan
- Cordipin
- Corinfar
- Corotrend
- Corynphar
- Depin
- Dignokonstant
- Dilafed
- Dilcor
- Dipinkor
- Duranifin
- Ecodipi
- Ecodipin
- Ecodipin E
- Fedcor
- Fedcor Retard
- Fenamon
- Fenamon Sr
- Fenihidin
- Fenihidine
- Glopir
- Hadipin
- Hexadilat
- Introcar
- Kordafen
- Macorel
- Megalat
- Myogard
- N1fedilat
- Nedipin
- Nicardia
- Nifangin
- Nifar
- Nifdemin
- Nifebene
- Nifecard
- Nifecor
- Nifedepat
- Nifedicor
- Nifedin
- Nifedine
- Nifedipine Retard
- Nifedipres
- Nifedirex LP
- Nifelan
- Nifelat
- Nifelat Q
- Nifelate
- Nifensar XL
- Nificard
- Nifidine
- Nifipen
- Niphedipine
- Orix
- Oxcord
- Pidilat
- Procardia
- Procardia XL
- Sepamit
- Tibricol
- Zenusin
Corynphar Brand Names Mixture
- No information avaliable
Corynphar Chemical_Formula
C17H18N2O6
Corynphar RX_link
http://www.rxlist.com/cgi/generic2/nifedip.htm
Corynphar fda sheet
Corynphar msds (material safety sheet)
Corynphar Synthesis Reference
Bossert, Vater, U.S. Pat. 3,485,847 (1969)
Corynphar Molecular Weight
346.335 g/mol
Corynphar Melting Point
172 - 174 oC
Corynphar H2O Solubility
Insoluble
Corynphar State
Solid
Corynphar LogP
2.343
Corynphar Dosage Forms
Capsule; Tablet; Tablet (extended-release)
Corynphar Indication
For the management of vasospastic angina, chronic stable angina and hypertension.
Corynphar Pharmacology
Nifedipine, the prototype of the dihydropyridine class of calcium-channel antagonists, is similar to other dihydropyridines including amlodipine, felodipine, isradipine, and nicardipine. Nifedipine is used to treat Prinzmetal's angina, hypertension, and other vascular disorders such as Raynaud's phenomenon. By blocking the calcium channels, Nifedipine inhibits the spasm of the coronary artery and dilates the systemic arteries, results in a increase of myocardial oxygen supply and a decrease in systemic blood pressure.
Corynphar Absorption
Rapidly and fully absorbed following oral administration.
Corynphar side effects and Toxicity
Symptoms of overdose include dizziness, drowsiness, nausea, severe drop in blood pressure, slurred speech, and weakness. LD50=494 mg/kg (orally in mice); LD50=1022 mg/kg (orally in rats)
Corynphar Patient Information
Corynphar Organisms Affected
Humans and other mammals