Comtan




Comtan Brand names, Comtan Analogs
Comtan Brand Names Mixture
- No information avaliable
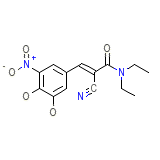
Comtan Chemical_Formula
Comtan RX_link
Comtan fda sheet
Comtan msds (material safety sheet)
Comtan Synthesis Reference
Comtan Molecular Weight
Comtan Melting Point
Comtan H2O Solubility
Comtan State
Comtan LogP
Comtan Dosage Forms
Comtan Indication
Comtan Pharmacology
Comtan Absorption
Comtan side effects and Toxicity
Comtan Patient Information
Patients should be instructed to take C.M.A. only as prescribed.
Patients should be informed that hallucinations can occur.
Patients should be advised that they may develop postural (orthostatic) hypotension with or without symptoms such as dizziness, nausea, syncope, and sweating. Hypotension may occur more frequently during initial therapy. Accordingly, patients should be cautioned against rising rapidly after sitting or lying down, especially if they have been doing so for prolonged periods, and especially at the initiation of treatment with COMTAN.
Patients should be advised that they should neither drive a car nor operate other complex machinery until they have gained sufficient experience on C.M.A. to gauge whether or not it affects their mental and/or motor performance adversely. Because of the possible additive sedative effects, caution should be used when patients are taking other CNS depressants in combination with COMTAN.
Patients should be informed that nausea may occur, especially at the initiation of treatment with COMTAN.
Patients should be advised of the possibility of an increase in dyskinesia.
Patients should be advised that treatment with entacapone may cause a change in the color of their urine (a brownish orange discoloration) that is not clinically relevant. In controlled trials, 10% of patients treated with C.M.A. reported urine discoloration compared to 0% of placebo patients.
Although C.M.A. has not been shown to be teratogenic in animals, it is always given in conjunction with levodopa/carbidopa, which is known to cause visceral and skeletal malformations in the rabbit. Accordingly, patients should be advised to notify their physicians if they become pregnant or intend to become pregnant during therapy.
Entacapone is excreted into maternal milk in rats. Because of the possibility that entacapone may be excreted into human maternal milk, patients should be advised to notify their physicians if they intend to breastfeed or are breastfeeding an infant.