Cognex




Cognex Brand names, Cognex Analogs
Cognex Brand Names Mixture
- No information avaliable
Cognex Chemical_Formula
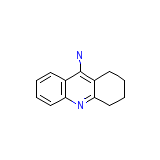
C13H14N2
Cognex RX_link
http://www.rxlist.com/cgi/generic2/tacrine.htm
Cognex fda sheet
Cognex msds (material safety sheet)
Cognex Synthesis Reference
No information avaliable
Cognex Molecular Weight
198.264 g/mol
Cognex Melting Point
183.5 oC
Cognex H2O Solubility
217 mg/L
Cognex State
Solid
Cognex LogP
2.298
Cognex Dosage Forms
No information avaliable
Cognex Indication
For the treatment of mild to moderate dementia of the Alzheimer's type.
Cognex Pharmacology
Tacrine is a parasympathomimetic, specifically, a reversible cholinesterase inhibitor. Tacrine is indicated for the treatment of mild to moderate dementia of the Alzheimer's type. Tacrine is postulated to exert its therapeutic effect by enhancing cholinergic function. This is accomplished by increasing the concentration of acetylcholine through reversible inhibition of its hydrolysis by acetylcholinesterase. If this proposed mechanism of action is correct, tacrine's effect may lessen as the disease process advances and fewer cholinergic neurons remain functionally intact. There is no evidence that tacrine alters the course of the underlying dementing process.
Cognex Absorption
Tacrine is rapidly absorbed. Absolute bioavailability of tacrine is approximately 17 ± 13%.
Cognex side effects and Toxicity
No information avaliable
Cognex Patient Information
No information avaliable
Cognex Organisms Affected
Humans and other mammals