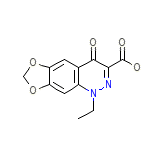
Cinobac




Cinobac Brand names, Cinobac Analogs
Cinobac Brand Names Mixture
- No information avaliable
Cinobac Chemical_Formula
C12H10N2O5
Cinobac RX_link
http://www.rxlist.com/cgi/generic3/cinobac.htm
Cinobac fda sheet
Cinobac msds (material safety sheet)
Cinobac Synthesis Reference
No information avaliable
Cinobac Molecular Weight
262.218 g/mol
Cinobac Melting Point
261 oC
Cinobac H2O Solubility
No information avaliable
Cinobac State
Solid
Cinobac LogP
0.617
Cinobac Dosage Forms
Capsules (250mg and 500mg)
Cinobac Indication
For the treatment of initial and recurrent urinary tract infections in adults caused by the following susceptible microorganisms: Escherichia coli, Proteus mirabilis, Proteus vulgaris, Klebsiella species (including K. pneumoniae), and Enterobacter species.
Cinobac Pharmacology
Cinoxacin is a synthetic antibacterial agent with in vitro activity against many gram-negative aerobic bacteria, particularly strains of the Enterobacteriaceae family. Cinoxacin inhibits bacterial deoxyribonucleic acid (DNA) synthesis, is bactericidal, and is active over the entire urinary pH range. Cross resistance with nalidixic acid has been demonstrated.
Cinobac Absorption
Rapidly absorbed after oral administration. The presence of food delays absorption but does does not affect total absorption.
Cinobac side effects and Toxicity
Oral, subcutaneous, and intravenous LD50 in the rat is 3610 mg/kg, 1380 mg/kg, and 860 mg/kg, respectively. Oral, subcutaneous, and intravenous LD50 in the mouse is 2330 mg/kg, 900 mg/kg, and 850 mg/kg, respectively.Symptoms following an overdose of cinoxacin may include anorexia, nausea, vomiting, epigastric distress, and diarrhea. The severity of the epigastric distress and the diarrhea are dose related. Headache, dizziness, insomnia, photophobia, tinnitus, and a tingling sensation have been reported in some patients.
Cinobac Patient Information
Cinobac Organisms Affected
Enteric bacteria and other eubacteria