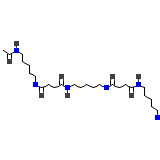
Cholestabyl




Cholestabyl Brand names, Cholestabyl Analogs
Cholestabyl Brand Names Mixture
- No information avaliable
Cholestabyl Chemical_Formula
C25H48N6O8
Cholestabyl RX_link
No information avaliable
Cholestabyl fda sheet
Cholestabyl msds (material safety sheet)
Cholestabyl Synthesis Reference
No information avaliable
Cholestabyl Molecular Weight
560.684 g/mol
Cholestabyl Melting Point
138-140oC
Cholestabyl H2O Solubility
1.2 mg/mL (at 20°C, 1.2%)
Cholestabyl State
Solid
Cholestabyl LogP
-0.478
Cholestabyl Dosage Forms
Powder (vials of 500 mg or 2 g sterile powder for preparing injections or for oral use)
Cholestabyl Indication
Used to treat acute iron or aluminum toxicity (an excess of aluminum in the body) in certain patients. Also used in certain patients with anemia who must receive many blood transfusions.
Cholestabyl Pharmacology
Deferoxamine, otherwise known as desferrioxamine or desferal, is a chelating agent used to remove excess iron or aluminum from the body. It acts by binding free iron or aluminum in the bloodstream and enhancing its elimination in the urine. By removing excess iron or aluminum, the agent reduces the damage done to various organs and tissues, such as the liver.
Cholestabyl Absorption
Deferoxamine is rapidly absorbed after intramuscular or subcutaneous administration, but only poorly absorbed from the gastrointestinal tract in the presence of intact mucosa.
Cholestabyl side effects and Toxicity
Intravenous LD50 in mouse, rat, and rabbit is 340 mg/kg, 520 mg/kg, and 600 mg/kg, respectively. Subcutaneous LD50 in mouse and rat is 1600 mg/kg and >1000 mg/kg, respectively. Oral LD50 in mouse and rat is >3000 mg/kg and >1000 mg/kg, respectively. Nephrotoxicity, ototoxicity and retinal toxicity have been reported following long-term administration for chronic iron overload.
Cholestabyl Patient Information
Cholestabyl Organisms Affected
Humans and other mammals