Chloromycetin Hydrocortisone




Chloromycetin Hydrocortisone Brand names, Chloromycetin Hydrocortisone Analogs
- Ak-Chlor Ophthalmic Ointment
- Ak-Chlor Ophthalmic Solution
- Ak-chlor
- Alficetyn
- Ambofen
- Amphenicol
- Amphicol
- Amseclor
- Anacetin
- Aquamycetin
- Austracil
- Austracol
- Biocetin
- Biophenicol
- CAF
- CAM
- CAP
- CPh
- Catilan
- Chemicetin
- Chemicetina
- Chlomin
- Chlomycol
- Chlora-Tabs
- Chloracol Ophthalmic Solution
- Chloramex
- Chloramfenikol
- Chloramficin
- Chloramfilin
- Chloramphenicol Sodium Succinate
- Chloramphenicole
- Chloramsaar
- Chlorasol
- Chloricol
- Chlornitromycin
- Chloro-25 vetag
- Chloroamphenicol
- Chlorocaps
- Chlorocid
- Chlorocid S
- Chlorocide
- Chlorocidin C
- Chlorocidin C tetran
- Chlorocol
- Chlorofair
- Chlorofair Ophthalmic Ointment
- Chlorofair Ophthalmic Solution
- Chloroject L
- Chloromax
- Chloromycetin
- Chloromycetin Hydrocortisone
- Chloromycetin Ophthalmic Ointment
- Chloromycetin Palmitate
- Chloromycetin for Ophthalmic Solution
- Chloromycetny
- Chloromyxin
- Chloronitrin
- Chloroptic
- Chloroptic Ophthalmic Solution
- Chloroptic S.O.P.
- Chloroptic-P S.O.P.
- Chlorovules
- Cidocetine
- Ciplamycetin
- Cloramfen
- Cloramficin
- Cloramicol
- Cloramidina
- Cloroamfenicolo
- Clorocyn
- Cloromisan
- Clorosintex
- Comycetin
- Cylphenicol
- D-Chloramphenicol
- Desphen
- Detreomycin
- Detreomycine
- Dextromycetin
- Doctamicina
- Econochlor
- Econochlor Ophthalmic Ointment
- Econochlor Ophthalmic Solution
- Elase-Chloromycetin
- Embacetin
- Emetren
- Enicol
- Enteromycetin
- Erbaplast
- Ertilen
- Farmicetina
- Farmitcetina
- Fenicol
- Fenicol Ophthalmic Ointment
- Globenicol
- Glorous
- Halomycetin
- Hortfenicol
- I-Chlor Ophthalmic Solution
- Intramycetin
- Isicetin
- Ismicetina
- Isophenicol
- Isopto fenicol
- Juvamycetin
- Kamaver
- Kemicetina
- Kemicetine
- Klorita
- Klorocid S
- Leukamycin
- Leukomyan
- Leukomycin
- Levomicetina
- Levomitsetin
- Levomycetin
- Loromisan
- Loromisin
- Mastiphen
- Mediamycetine
- Medichol
- Micloretin
- Micochlorine
- Micoclorina
- Microcetina
- Mychel
- Mychel-Vet
- Mychel-s
- Mycinol
- Normimycin V
- Novochlorocap
- Novomycetin
- Novophenicol
- Ocu-Chlor Ophthalmic Ointment
- Ocu-Chlor Ophthalmic Solution
- Oftalent
- Oleomycetin
- Opclor
- Opelor
- Ophtho-Chloram Ophthalmic Solution
- Ophthochlor
- Ophthochlor Ophthalmic Solution
- Ophthoclor
- Ophthocort
- Ophtochlor
- Optomycin
- Otachron
- Otophen
- Owadziak
- Pantovernil
- Paraxin
- Pedraczak
- Pentamycetin
- Pentamycetin Ophthalmic Ointment
- Pentamycetin Ophthalmic Solution
- Pflanzol
- Quellada
- Quemicetina
- Rivomycin
- Romphenil
- Ronphenil
- Sang-«gamma»
- Septicol
- Sificetina
- Sintomicetina
- Sintomicetine R
- Sno-Phenicol
- Sopamycetin Ophthalmic Ointment
- Sopamycetin Ophthalmic Solution
- Spectro-Chlor Ophthalmic Ointment
- Spectro-Chlor Ophthalmic Solution
- Stanomycetin
- Synthomycetin
- Synthomycetine
- Synthomycine
- Tega-Cetin
- Tevcocin
- Tevcosin
- Tifomycin
- Tifomycine
- Tiromycetin
- Treomicetina
- Tyfomycine
- Unimycetin
- Veticol
- Viceton
Chloromycetin Hydrocortisone Brand Names Mixture
- No information avaliable
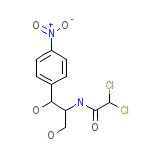
Chloromycetin Hydrocortisone Chemical_Formula
C11H12Cl2N2O5
Chloromycetin Hydrocortisone RX_link
http://www.rxlist.com/cgi/generic3/chloramphenicol.htm
Chloromycetin Hydrocortisone fda sheet
Chloromycetin Hydrocortisone msds (material safety sheet)
Chloromycetin Hydrocortisone Synthesis Reference
No information avaliable
Chloromycetin Hydrocortisone Molecular Weight
323.129 g/mol
Chloromycetin Hydrocortisone Melting Point
150.5 oC
Chloromycetin Hydrocortisone H2O Solubility
2500 mg/L (at 25 °C)
Chloromycetin Hydrocortisone State
Solid
Chloromycetin Hydrocortisone LogP
1.476
Chloromycetin Hydrocortisone Dosage Forms
Capsule; Drops; Liquid; Ointment; Powder; Solution; Suspension; Tablet
Chloromycetin Hydrocortisone Indication
Used in treatment of cholera, as it destroys the vibrios and decreases the diarrhea. It is effective against tetracycline-resistant vibrios. It is also used in eye drops or ointment to treat bacterial conjunctivitis.
Chloromycetin Hydrocortisone Pharmacology
Chloramphenicol is a broad-spectrum antibiotic that was derived from the bacterium Streptomyces venezuelae and is now produced synthetically. Chloramphenicol is effective against a wide variety of microorganisms, but due to serious side-effects (e.g., damage to the bone marrow, including aplastic anemia) in humans, it is usually reserved for the treatment of serious and life-threatening infections (e.g., typhoid fever). Chloramphenicol is bacteriostatic but may be bactericidal in high concentrations or when used against highly susceptible organisms. Chloramphenicol stops bacterial growth by binding to the bacterial ribosome (blocking peptidyl transferase) and inhibiting protein synthesis.
Chloromycetin Hydrocortisone Absorption
Rapidly and completely absorbed from gastrointestinal tract following oral administration (bioavailability 80%). Well absorbed following intramuscular administration (bioavailability 70%). Intraocular and some systemic absorption also occurs after topical application to the eye.
Chloromycetin Hydrocortisone side effects and Toxicity
Oral, mouse: LD50 = 1500 mg/kg; Oral, rat: LD50 = 2500 mg/kg. Toxic reactions including fatalities have occurred in the premature and newborn; the signs and symptoms associated with these reactions have been referred to as the gray syndrome. Symptoms include (in order of appearance) abdominal distension with or without emesis, progressive pallid cyanosis, vasomotor collapse frequently accompanied by irregular respiration, and death within a few hours of onset of these symptoms.
Chloromycetin Hydrocortisone Patient Information
No information avaliable
Chloromycetin Hydrocortisone Organisms Affected
Enteric bacteria and other eubacteria