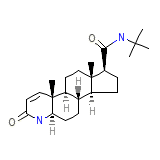
Chibro-Proscar




Chibro-Proscar Brand names, Chibro-Proscar Analogs
Chibro-Proscar Brand Names Mixture
- No information avaliable
Chibro-Proscar Chemical_Formula
C23H36N2O2
Chibro-Proscar RX_link
http://www.rxlist.com/cgi/generic3/propecia.htm
Chibro-Proscar fda sheet
Chibro-Proscar msds (material safety sheet)
Chibro-Proscar Synthesis Reference
No information avaliable
Chibro-Proscar Molecular Weight
372.544 g/mol
Chibro-Proscar Melting Point
252-254 oC
Chibro-Proscar H2O Solubility
11.7 mg/L
Chibro-Proscar State
Solid
Chibro-Proscar LogP
4.277
Chibro-Proscar Dosage Forms
Tablet
Chibro-Proscar Indication
For the treatment of symptomatic benign prostatic hyperplasia (BPH) in men with an enlarged prostate to: Improve symptoms, reduce the risk of acute urinary retention, reduce the risk of the need for surgery including transurethral resection of the prostat.
Chibro-Proscar Pharmacology
Finasteride is indicated for the treatment of male pattern hair loss (androgenetic alopecia) in men only. Finasteride is a competitive and specific inhibitor of Type II 5a-reductase, an intracellular enzyme that converts the androgen testosterone into DHT. Two distinct isozymes are found in mice, rats, monkeys, and humans: Type I and II. Each of these isozymes is differentially expressed in tissues and developmental stages. In humans, Type I 5a-reductase is predominant in the sebaceous glands of most regions of skin, including scalp, and liver. Type I 5a-reductase is responsible for approximately one-third of circulating DHT. The Type II 5a-reductase isozyme is primarily found in prostate, seminal vesicles, epididymides, and hair follicles as well as liver, and is responsible for two-thirds of circulating DHT.
Chibro-Proscar Absorption
No information avaliable
Chibro-Proscar side effects and Toxicity
No information avaliable
Chibro-Proscar Patient Information
No information avaliable
Chibro-Proscar Organisms Affected
Humans and other mammals